-
- Posts: 15
- Joined: Sun Jan 18, 2009 5:00 pm
Cappillary column
Splitless inlet
Pressure 8.48
Temp 220C
Aerage velocity 179 cm/ sec
Carrier gas H2 flow 40
Makeup gas N2 35
Air 400
Injection volume1 ul.
Standard in water.
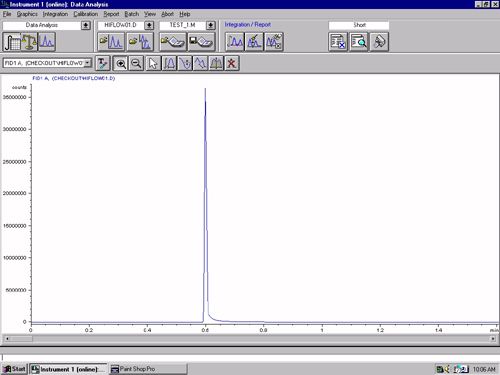
Standard: Ethylene oxide and Ethylene chlorohydrins. Diluted to get 100-1ppm
Concern: gauscian peaks for all concentrations but peak tailing is >2.0 and it should be <1.8. Interesting fact is peak for EO is > 2 and peak for ECH is <1.2. Both come out insame injection at ~ 0.407sec and 3.25 mins.
Installed new column. Baseline is perfect. Blank water samples noooooooo peaks. Changed inlet (900ul) septa.
Any suggestion to reduce peak tailing.