-
- Posts: 23
- Joined: Fri Aug 09, 2013 4:02 pm
Please help me!!!
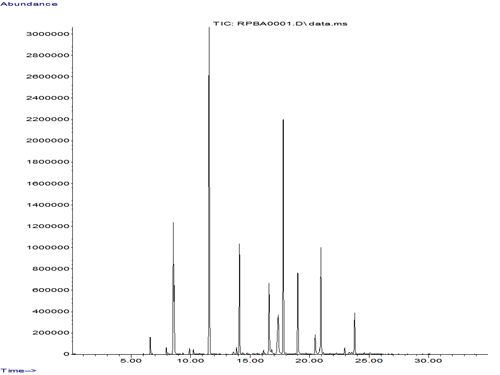
I am using CAR/DVB/PDMS 50/30um- SPME fiber. I am analysing the beer samples and to obtain calibration curves I applied this device in the headspace of standards mixture (ethanol, acetaldehyde, ethyl acetate, propanol, isobutanol, isoamyl alcohol, isoamyl acetate). Unfortunately, I could not get expected chromatogram with SPME fiber. The component were not well separated. Before this procedure I directly injected the mixture of the same compounds and got normally separated peaks in my column. I know that this fiber (CAR/DVB/PDMS 50/30um) is not appropriate for my aroma extraction but at least it is suitable for ethanol extraction. Afterwards, I prepared the ethanol solution just to observe the result and unfortunaly, I got a very long tailing peak. Could you tell me please what is the problem and how I can eliminate it? The GC conditions were: injector-250C, splitless mode, Detector-210C, Column-VF5MS (30x0.2x0.25), Oven- 40 to 120C with 10C/min, . The SPME conditions: 10min extraction at ambient temperature with stirring, 5 min desorption.
Could you tell me please how I can get normally separated peaks in chromatogram?
Best regards,
Ksenia