-
- Posts: 1
- Joined: Wed Oct 17, 2012 11:21 am
I am trying to separate the following structures with TLC. I have tried using 0.4M ammonium sulphate which is regularly used in the lab for TLC. But both the structures run side by side. Can someone please suggest any other solvents I can try. Looking for an educated guess.
Thank you.
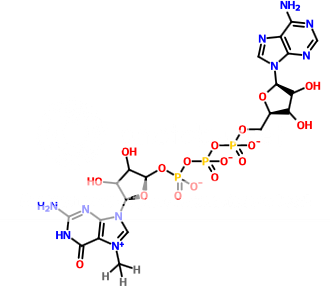
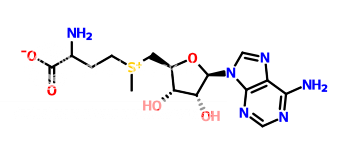
p.s. I am not a chemist and don't understand much about chemistry. Therefore your guess is probably much better than mine.
