-
- Posts: 2
- Joined: Fri Apr 29, 2011 8:51 am
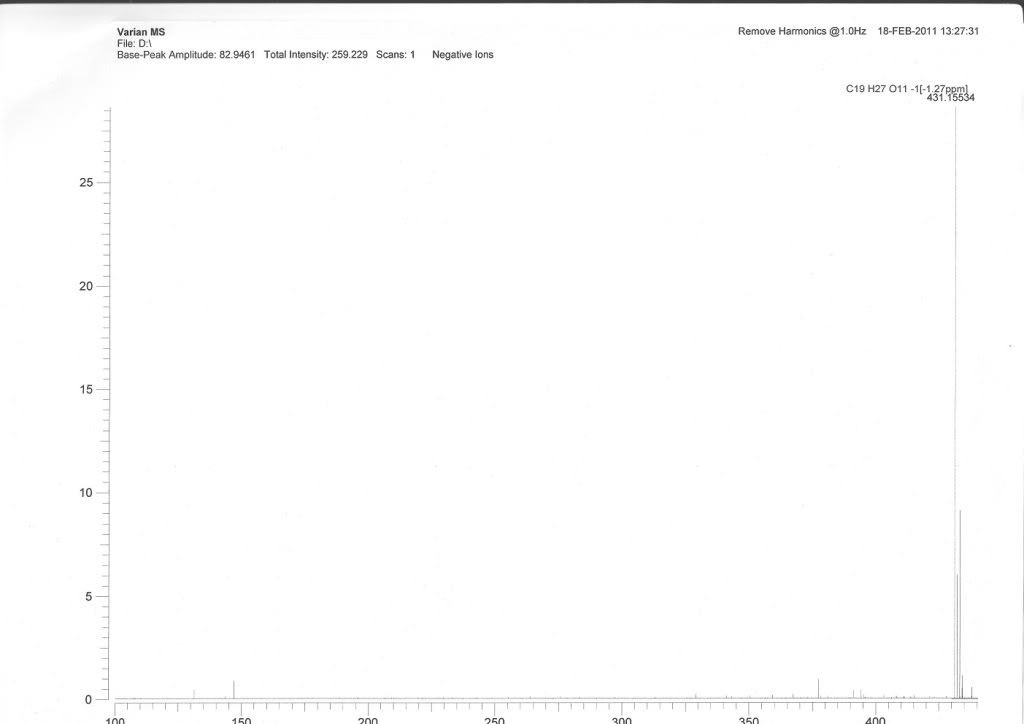
Thanks for help.
Advertisement
Discussions about GC-MS, LC-MS, LC-FTIR, and other "coupled" analytical techniques.
Separation Science offers free learning from the experts covering methods, applications, webinars, eSeminars, videos, tutorials for users of liquid chromatography, gas chromatography, mass spectrometry, sample preparation and related analytical techniques.
Subscribe to our eNewsletter with daily, weekly or monthly updates: Food & Beverage, Environmental, (Bio)Pharmaceutical, Bioclinical, Liquid Chromatography, Gas Chromatography and Mass Spectrometry.