-
- Posts: 93
- Joined: Tue Jul 15, 2008 6:59 am
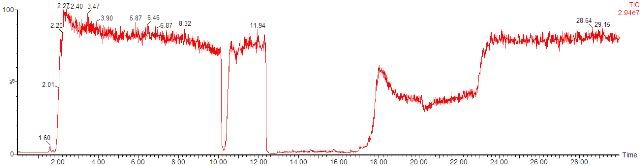

Would those tiny dips in baseline (e.g. a dip starting around 7.25 min in the second chromatogram) be considered as ion suppression due to matrix effect? The large dips are obvious, but to what extent should the suppression be, to be considered significant?
Blank milk extracts were injected. 100ppm cyanuric acid and 100ppm melamine were infused at 5 uL/min for each separate run. Is that infusion rate considered to be too low? It is obvious I am limited by the volume of the infusion syringe and analysis runtime, but what other parameters should I consider?
Pardon these elementary questions. I am not too sure what to expect, given my limited experience with LCMS. Thanks!
