-
- Posts: 527
- Joined: Fri Feb 10, 2012 7:39 pm
Advertisement
Discussions about GC-MS, LC-MS, LC-FTIR, and other "coupled" analytical techniques.




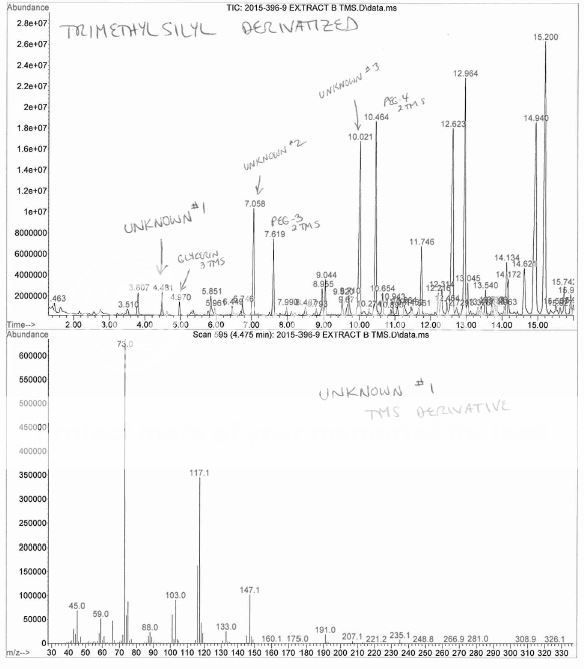
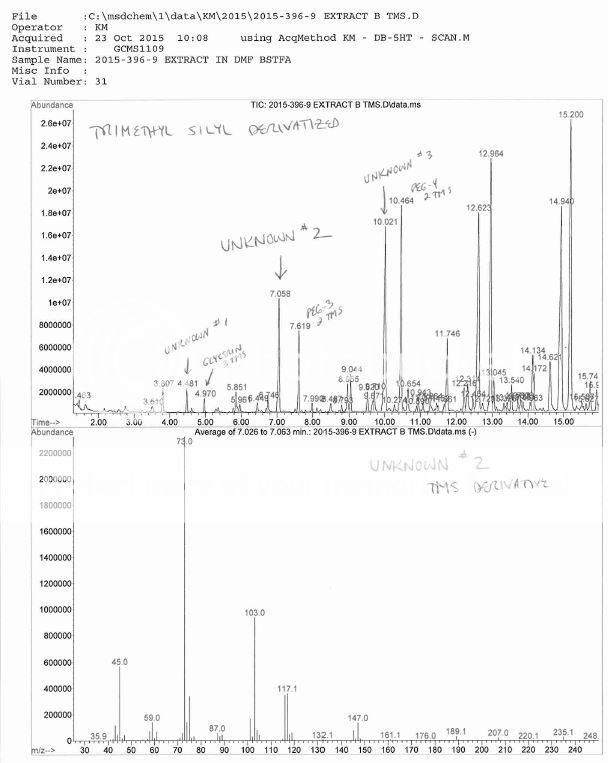
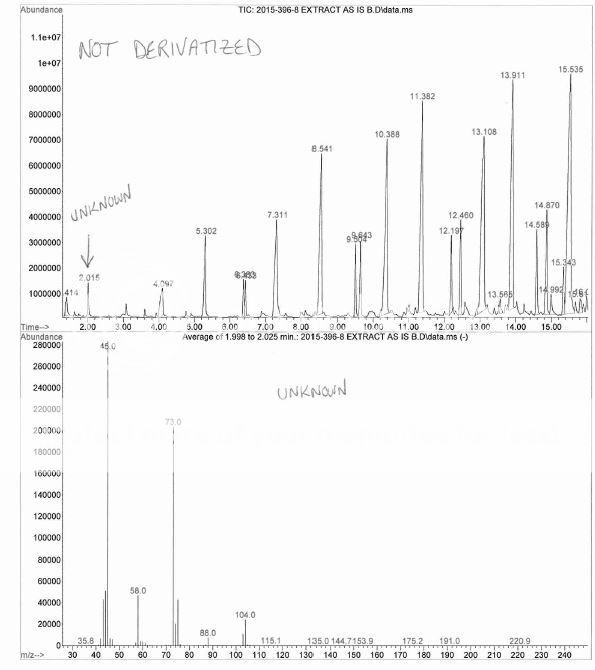
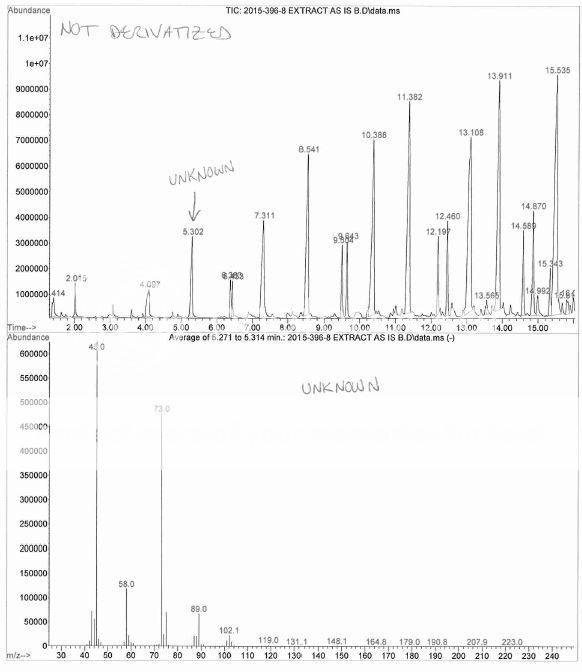
Ha ! My boss had to through in his 2 cents worth that I had overloaded the column, the sample was residue extracted then taken up in a few drops of DMF, then mixed with BSTFA, then injected overnight. No, I did NOT dilute more and re-inject, no reason too, this is a qualitative investigation.Nice Chromatography !, short column, fast temperature ramp, hydrogen maybe ?
I did send an E-mail. Yes, on Chemstation MSD. I believe you’ll need to make a Directory like UNKNOWN.D like on a flash drive and copy the attached files to that, then load up UNKNOWN.D into your Data Analysis. Thanks for the offer; I think if one gets identified that the series falls into place. I'm OK - if you have colleagues who are intrigued by stuff like this or have other search libraries - if you share the files.You can email the GC/MS data to myself sschoenfeld[at]innovaflavors.com and I'd be happy to see if my custom flavors and fragrances library gets a hit on the nonsilylated unknowns (I assume it was collected on Chemstation MSD).
Separation Science offers free learning from the experts covering methods, applications, webinars, eSeminars, videos, tutorials for users of liquid chromatography, gas chromatography, mass spectrometry, sample preparation and related analytical techniques.
Subscribe to our eNewsletter with daily, weekly or monthly updates: Food & Beverage, Environmental, (Bio)Pharmaceutical, Bioclinical, Liquid Chromatography, Gas Chromatography and Mass Spectrometry.