by
ece » Fri Sep 07, 2007 6:54 pm
gotcha, we'll do that if we suspect a leak. last time i calibrated, all the lines except for triolein were +slope lines, with a correlation coefficient of .99 or better. triolein had a .98 but we are sure it's our column which was designed for lower temperatures than our method dictates for the final ramp, column bleed.
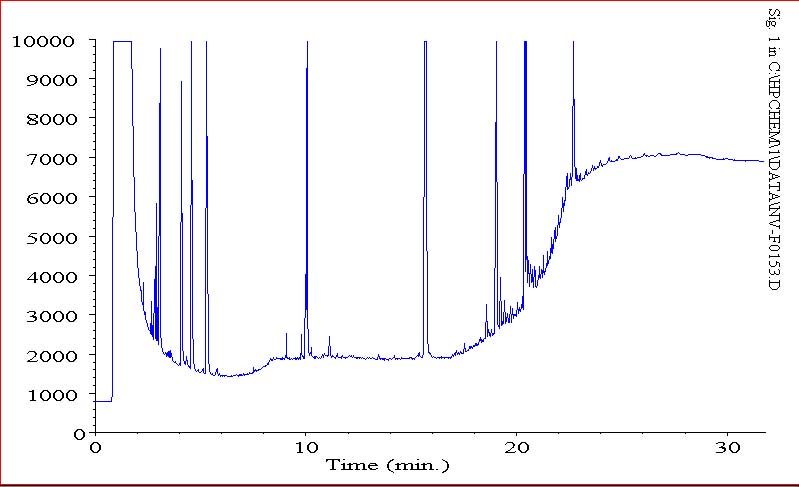
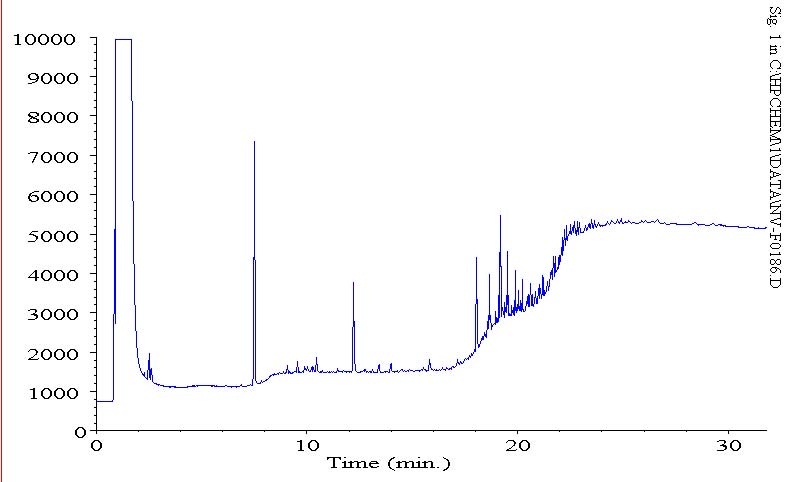
the following are three chromatograms(phs) that display the column bleed at the end (380 C), since our column is designed for 370 C (our salesman claimed there was no column available with a higher stat. phase thermal limit) i figured this was just column bleed.
this was an initial run of a calibration std
this was a later run of a calibration std
this is a recent run, an actual sample
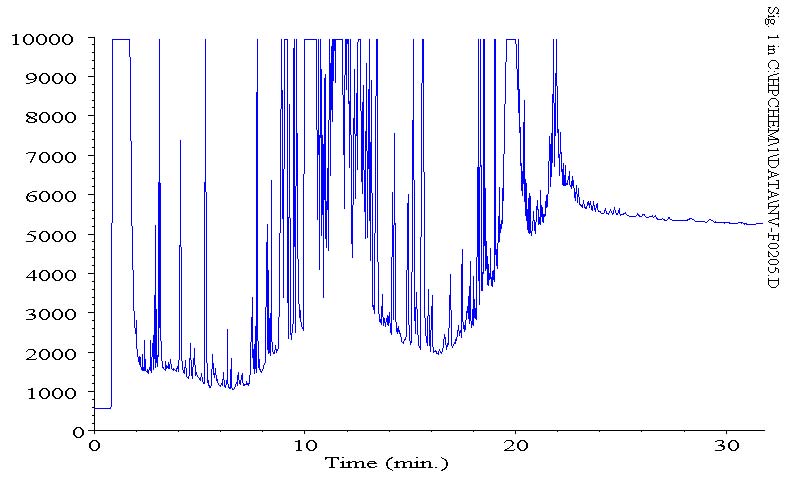
as you can see, lower bleed on a subsequent std of a calibration run, but also less intensity on the same standard. now that i am running actual samples, i am seeing less bleed. initially, we were seeing high column bleed.
i can come to a couple of conclusions on this:
1) the stationary phase in the column might be spent, although if it were spent then i probably wouldn't see any peaks or perhaps i would see a shift of the peaks' retention times, which isn't happening
2) samples (if they are on spec fuel) typically have lower concentrations of all of the glycerides than calibration standards, so the stationary phase retain fewer glycerides in an actual sample than in a calibration standard.
i am only concerned right now with the breakdown of the stationary phase to the point where the column becomes brittle and breaks. but since i am still seeing peaks where i am supposed to see them, i'm not too worried yet. fortunately, we are planning on investing in a more robust column appropriate for higher temperatures.
thanks a lot for your input as always, folks!



