-
- Posts: 9
- Joined: Wed Feb 16, 2011 3:35 pm
This is my first time using this forum, so please bear with me if my topic has already been covered. I searched for information regarding my issue, but couldn't find anything in previous posts. Hope someone has some insight!
So, the issue: (sorry for such along post...just wanted to be as detailed as possible) I am currently trying to separate and quantify various sugars in aqueous solution using a Benson Polymeric 806 BP-100 Ag+ Carbohydrate Column with 806G BP-100 Ag+ Carbohydrate Guard Column, operating at 90 degrees Celsius as recommended, and filtered, degassed water as mobile phase, running at 0.3 mL/min. We are using a Waters 2414 RI for detection.
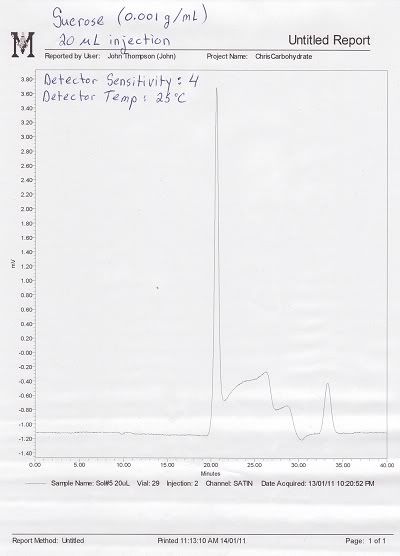
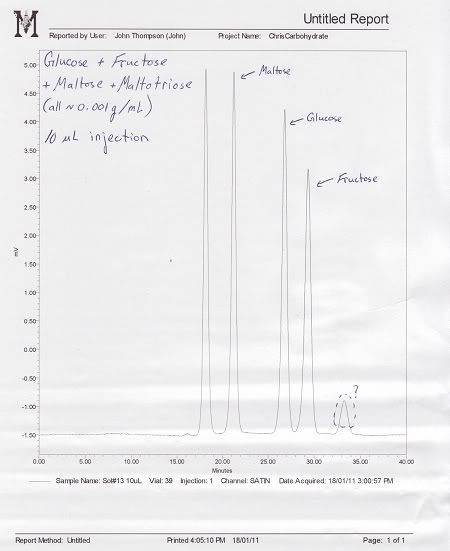
So far, I have had fairly good success in resolving well defined peaks using standard solutions of Maltose, Maltotriose, Glucose, and Fructose. I am also trying to separate Sucrose in order to obtain a standard curve for that sugar, however the peaks are not well defined. Sucrose consistently shows a sharp increase followed by a long, sloppy decline (see attached images). All the solutions I am running are roughly 0.001 g/mL concentration for comparative purposes.
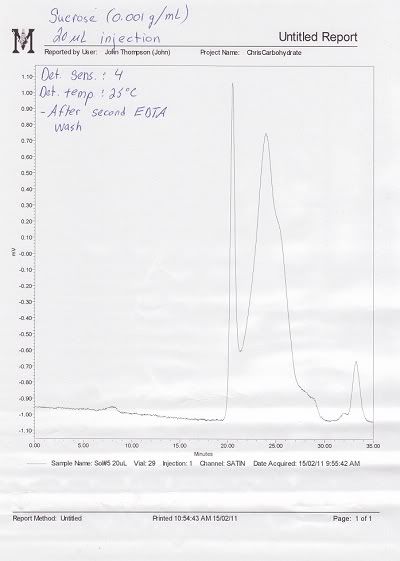
Please find attached two chromatograms of Sucrose alone illustrating what is happening with the peaks. The first illustrates the elution of Sucrose before I washed the column with a 0.01 M EDTA disodium dihydrate solution (under the recommendations of a BP technician), while the second illustrates how Sucrose elutes following the EDTA washing. It is puzzling why Sucrose does not resolve well, yet Maltose (a very similar disaccharide) resolves quite well.
I have tried a number of possible solutions, and nothing seems to resolve the peak better. I have tried ordering high purity sucrose (>=99.9%) and running that through, but the problem persists (the chromatograms attached for Sucrose are the >=99.9% sample). I have tried increasing and lowering concentration of my standard solutions, changing the flow rate of the system, we have even bought a brand new Waters differential refractometer (as our old one did not have the best sensitivity at even the highest concentrations) and the problem still persists. Despite the issues of sensitivity with our older RI detector, this phenomenon was still displayed, which makes me think it is not a setting on the detector which needs to be adjusted, especially considering all my other sugars separate fine. For these reasons I believe this to be a column problem.
Does anyone have any suggestions on what might be happening with the column, or why this separation of Sucrose looks so sloppy? We have been through all the troubleshooting guides we can find and still cannot find any solution.
Please advise, and thanks so much for taking the time to read this!
-Chris
p.s. I apologize for the size of the images... I tried saving them on my computer as about 400 x 400 pixels, however they still seem to be showing up quite large in the preview. I'd be very appreciative if anyone has any suggestions on how to prevent that as well!