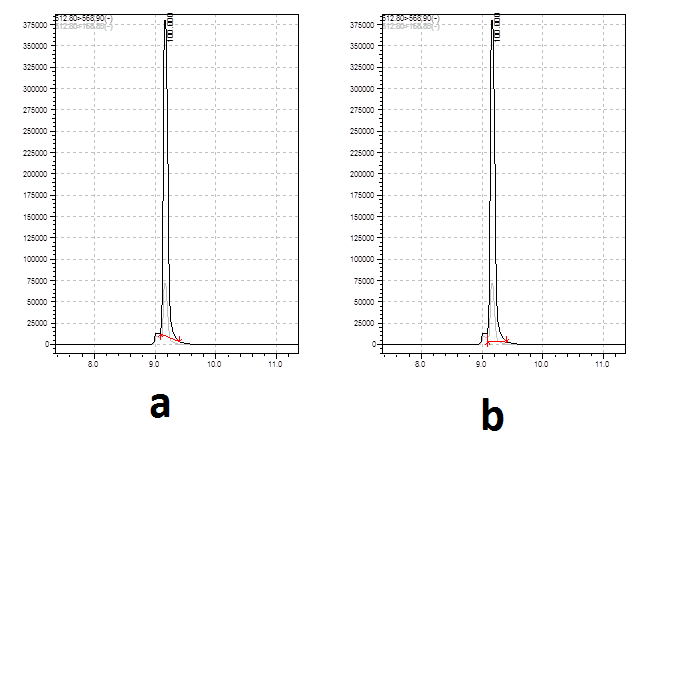
-
- Posts: 5
- Joined: Tue Mar 10, 2015 1:47 pm
Advertisement
Peak area selection
Discussions about HPLC, CE, TLC, SFC, and other "liquid phase" separation techniques.
11 posts
Page 1 of 1
-
- Posts: 5436
- Joined: Thu Oct 13, 2005 2:29 pm
Take your pick - by eye the relative difference between the two areas will be less than 2%. If that makes a practical difference then you need to get the peaks separated or find a selective wavelength.
As long as you do it the same way every time AND as long as the small peak in front of your analyte always stays about the same size your results will still be repeatable.
Peter
As long as you do it the same way every time AND as long as the small peak in front of your analyte always stays about the same size your results will still be repeatable.
Peter
Peter Apps
-
- Posts: 5
- Joined: Tue Mar 10, 2015 1:47 pm
Thank you for your reply, Peter. But in several cases the difference between two areas may reach 20%. Please refer to the pic posted below.Take your pick - by eye the relative difference between the two areas will be less than 2%. If that makes a practical difference then you need to get the peaks separated or find a selective wavelength.
As long as you do it the same way every time AND as long as the small peak in front of your analyte always stays about the same size your results will still be repeatable.
Peter
I wonder that which peak area selection method is more acceptable without separating the small peak in front of my analyte.
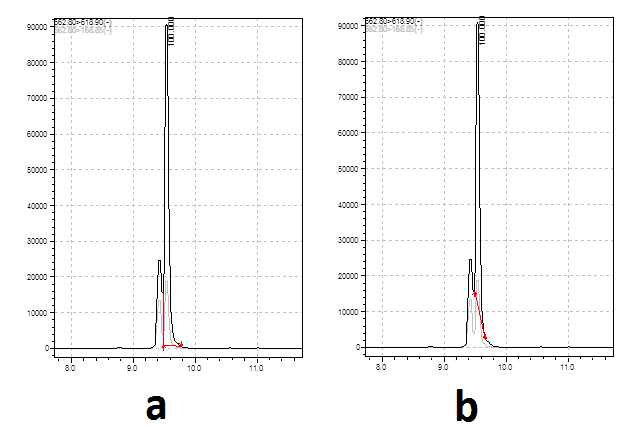
-
- Posts: 5436
- Joined: Thu Oct 13, 2005 2:29 pm
If the interference peak varies by that much then you have to go with A, but you really need to improve the chomatography - you have lots of space either side of these two peaks to shift them into.
Peter
Peter
Peter Apps
-
- Posts: 1408
- Joined: Thu Jul 28, 2005 2:08 pm
In my opinion, B in the first example and A in the second.
I think this is a great example/argument for the the above
Best Regards
I think this is a great example/argument for the the above
Best Regards
Learn Innovate and Share
Dancho Dikov
Dancho Dikov
-
- Posts: 115
- Joined: Tue Apr 19, 2005 12:36 pm
Hi goldforest12345
I'll try a gradient elution run to separate the first peak (impurity?, decomposition?) and then try to run isocratically. The integration criteria is correct .
Best regards.
Fernando
I'll try a gradient elution run to separate the first peak (impurity?, decomposition?) and then try to run isocratically. The integration criteria is correct .
Best regards.
Fernando
-
- Posts: 58
- Joined: Fri Nov 21, 2014 8:31 am
This is how the intergation of the peak should look like. I think only couple of chromatographic programs can do this.

If you have trouble with not separeted peaks you can always make a baseline and use peak height instead of peak area.
Kreall

If you have trouble with not separeted peaks you can always make a baseline and use peak height instead of peak area.
Kreall
-
- Posts: 5436
- Joined: Thu Oct 13, 2005 2:29 pm
I disagree I'm afraid. The reconstructed peak shape assumes that both peaks have an ideal shape, and is still vulnerable to changes in the size of the interfering peak. And if the target peak is riding on the tail of an interference than there is no way of knowing where the baseleine should be set.
Peter
Peter
Peter Apps
-
- Posts: 3600
- Joined: Mon Aug 30, 2004 11:43 pm
Here's my take:
You've got partial separation, so small changes may significantly help resolve the two peaks and make your question moot. And don't throw USP crap or "validated procedure", any robustness studies - if done correctly - document that small changes are acceptable.
If mine, first thing I would do is run at 10C lower temperature, and 10C higher temperature. The resolution will either be better, worse, or the same. If one temperature is better, explore that, and go from there. If both higher and lower do not help, then time to tweak a different parameter.
I recently did similar to separate two chelating agents that practically coeluted using our 20-year-old procedure; in that case, going to smaller particle size and increasing column temperature from 30C to 50C gave me baseline resolution.
You've got partial separation, so small changes may significantly help resolve the two peaks and make your question moot. And don't throw USP crap or "validated procedure", any robustness studies - if done correctly - document that small changes are acceptable.
If mine, first thing I would do is run at 10C lower temperature, and 10C higher temperature. The resolution will either be better, worse, or the same. If one temperature is better, explore that, and go from there. If both higher and lower do not help, then time to tweak a different parameter.
I recently did similar to separate two chelating agents that practically coeluted using our 20-year-old procedure; in that case, going to smaller particle size and increasing column temperature from 30C to 50C gave me baseline resolution.
-

- tom jupille
- Site Admin
-
- Posts: 4978
- Joined: Wed Aug 11, 2004 4:55 pm
Neither one is any better than guesswork. The only way to get an accurate result is to do a better job with the separation.
There's an illuminating couple of sentences in Dyson's book on integration:
“. . . errors arising from peak overlap are introduced by the algorithms of perpendicular and tangent separation and cannot be eliminated by anything but better chromatography. Integrators are able to generate a highly precise and totally inaccurate set of results for all the foregoing examples.”
Dyson, Chromatographic Integration Methods, 2nd ed. , pg 67; RSC Monographs (1998) (emphasis added)
There's an illuminating couple of sentences in Dyson's book on integration:
“. . . errors arising from peak overlap are introduced by the algorithms of perpendicular and tangent separation and cannot be eliminated by anything but better chromatography. Integrators are able to generate a highly precise and totally inaccurate set of results for all the foregoing examples.”
Dyson, Chromatographic Integration Methods, 2nd ed. , pg 67; RSC Monographs (1998) (emphasis added)
-- Tom Jupille
LC Resources / Separation Science Associates
tjupille@lcresources.com
+ 1 (925) 297-5374
LC Resources / Separation Science Associates
tjupille@lcresources.com
+ 1 (925) 297-5374
-
- Posts: 5
- Joined: Tue Mar 10, 2015 1:47 pm
Thank you guys so much for your precious comments!
Lam
Lam
11 posts
Page 1 of 1
Who is online
In total there are 8 users online :: 1 registered, 0 hidden and 7 guests (based on users active over the past 5 minutes)
Most users ever online was 11462 on Mon Dec 08, 2025 9:32 pm
Users browsing this forum: Ahrefs [Bot] and 7 guests
Most users ever online was 11462 on Mon Dec 08, 2025 9:32 pm
Users browsing this forum: Ahrefs [Bot] and 7 guests
Latest Blog Posts from Separation Science
Separation Science offers free learning from the experts covering methods, applications, webinars, eSeminars, videos, tutorials for users of liquid chromatography, gas chromatography, mass spectrometry, sample preparation and related analytical techniques.
Subscribe to our eNewsletter with daily, weekly or monthly updates: Food & Beverage, Environmental, (Bio)Pharmaceutical, Bioclinical, Liquid Chromatography, Gas Chromatography and Mass Spectrometry.
- Follow us on Twitter: @Sep_Science
- Follow us on Linkedin: Separation Science