Page 1 of 1
Extraction of fragrances from lotions / Moisturizers
Posted: Fri Mar 28, 2014 3:55 pm
by mleee
Hi all,
I am trying to analyze fragrance mixtures in some of lotions / moisturizers. I have been done extractions with Pentane and Hexane. I have run the extracted sample in the GCMS but then some of base materials including parabens are also extracted along with the aroma chemicals .. which covers some of the peaks and would not allow me to give readings that I want.
Is there any other ways to do better fragrance extractions from the lotions / moisturizers?
Re: Extraction of fragrances from lotions / Moisturizers
Posted: Fri Mar 28, 2014 5:51 pm
by rb6banjo
Seems like a classic headspace analysis. The active ingredients for the "lotion" part of the formulation will not be nearly as volatile as those that make up the fragrance package. Put some in a vial and analyze the headspace using solid-phase microextraction. Should do the trick!
My experience has been that if you want quantitative information from real samples like this using SPME, you should calibrate in your matrix (method of standard additions). Good luck.
Re: Extraction of fragrances from lotions / Moisturizers
Posted: Fri Mar 28, 2014 7:42 pm
by KM-USA
I am trying to analyze fragrance mixtures in some of lotions / moisturizers.....some of base materials including parabens are also extracted along with the aroma chemicals .. which covers some of the peaks and would not allow me to give readings that I want. Is there any other ways to do better fragrance extractions from the lotions / moisturizers?
Are you trying to determine how much fragrance you added to your own product, trying to find out which fragrance components are in a competitive product, trying to see if some fragrance components have volatilized or degraded, or trying to guess at the level of fragrance in a competitive product? That could be four different techniques.
Maybe the Consumer Products Guy will respond, he's got tons of experience with stuff like this.
Re: Extraction of fragrances from lotions / Moisturizers
Posted: Fri Mar 28, 2014 11:46 pm
by MSCHemist
Quantitation with SPME can be tricky depending on the analytes. Some have precission issues, some have linearity issues, and everything effects binding to the fiber. I once tried making a calibration mix in acetone instead of water which added only 3ul of acetone to a 2ml total volume and I saw a 30% drop in the earliest half of the chromatogram through about 1400 kovats. Still it is the best alternative.
I do a lot of trapped flavors and fragrances. Today I was given some inclusions which are i fiber and suspended in fat. I tried dissolving in ethyl acetate warmed to 55 deg. I had to inject 2ul pulse splitless from 2g to 3ml ethyl acetate to see peaks.
Re: Extraction of fragrances from lotions / Moisturizers
Posted: Sun Mar 30, 2014 6:07 pm
by rb6banjo
Did a couple of quick things on Friday with the hand soap from the men's room close to my office. Here are the results. Neat soap (200 mg soap in 22 mL headspace vial, 30 minutes, SPME with carboxen/pdms/dvb-2cm, RT):
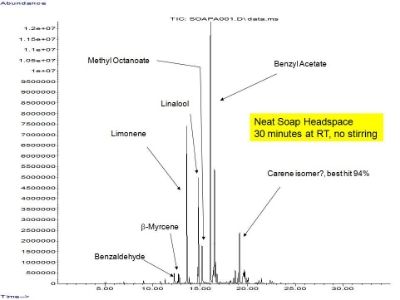
Cut the soap with water (200 mg to 1.04 g with water, stirred the sample, same SPME conditions). Blue trace is the aqueous mix. Black trace is the same as shown in the other graphic.
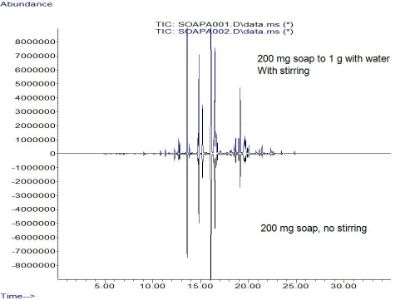
You must take care in your calibration but quantitative analysis is possible. The sensitivity is better for the 200 mg sample cut with water but stirred.
Re: Extraction of fragrances from lotions / Moisturizers
Posted: Mon Mar 31, 2014 8:19 pm
by rb6banjo
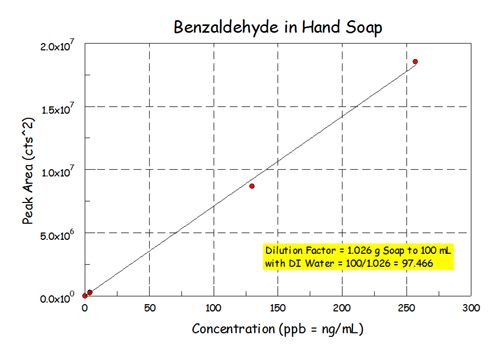
Please don't be afraid of SPME as a quantitative method. I have had very good success in my career using this technique. Here's what I got for benzaldehyde in that soap.
I used the method of standard addition after diluting the soap 1 g to 100 mL with DI water. I determined about 3.6 ppb +/- 0.2 ppb benzaldehyde in the dilute sample. The 95% confidence limit of that result is +/- 0.6 ppb. Using the dilution factor the concentration in the soap is 0.35 ppm (µg/g) +/- 0.06 ppm (95% confidence). I also used heated headspace to perform the analysis. I see lots more "stuff" when I heat the sample a bit.


