-
- Posts: 2
- Joined: Tue Nov 26, 2013 1:06 pm
This is my first post over here. I'm quite new on chromatography and found this very helpful site.
I'm working with Agilent GC 7820A with FID and autosampler. We are using N2 as carrier gas and HP-5 column (15m).
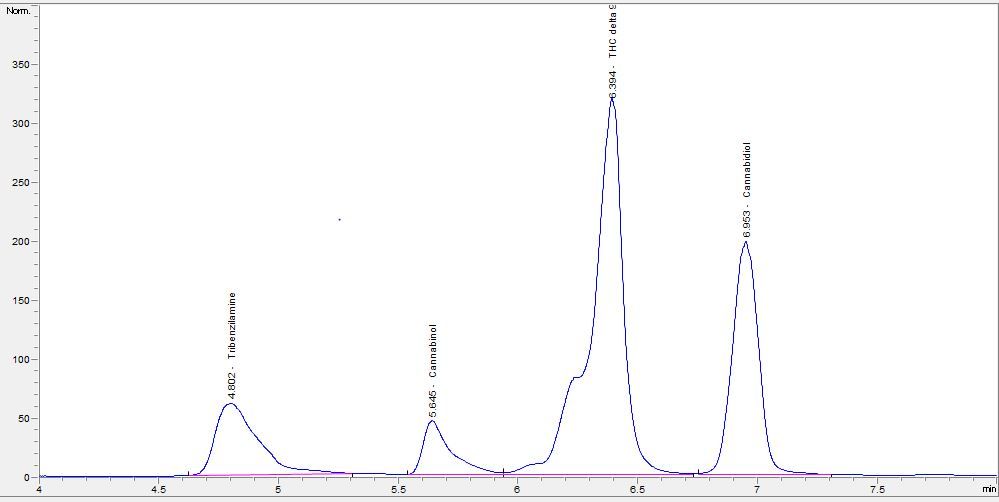
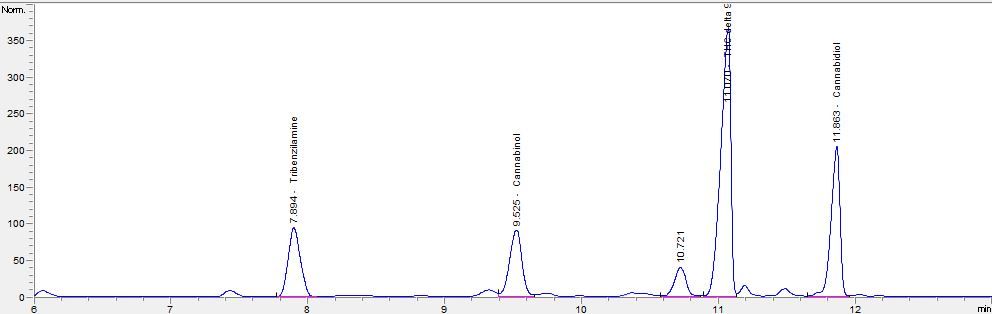
Our work is based on cannabinoids.
I have some problem separating peaks of delta8 and delta9 THC due to its very close retention time.
Recently, I found a document where they suggest a Rxi®-5ms column and hidrogen as carrier gas.
http://blog.restek.com/?p=7704
So, my questions are:
- Will I improve my analysis changing the column for Rxi®-5ms (same lenght, thickness and diameter)? On description, it says tht is virtually equal to HP-5. According to despcription the column is specific for MS!
- Will H2 as carrier improve my analysis aswell?
- I'm using H2 generator for FID. Can use it for both FID and carrier gas?
Thanks in advanced.