Page 1 of 2
5975 Sensitivity questions..
Posted: Wed Jun 29, 2011 7:37 pm
by cjm
Here's the latest of my many issues, I'm running a 1 ppb standard of all 209 pcb congeners. ms is in sim mode. the following chromatogram shows great peak shape up until 63 minutes, when all hell breaks loose. This does not happen every run, some runs are flawless, others are useless as this one is. Total run time is 109minutes so this event took place 40 minutes before the end of the run. I believe something is very very wrong with the mass spec. we've taken it apart and cleaned it. this happens....any thoughts as to what may cause this? Also..sometimes it "recovers" and peak shape suddenly returns.
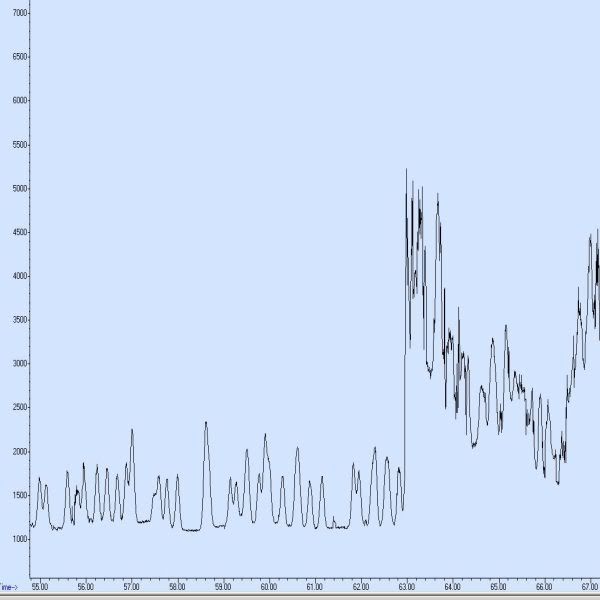
Re: 5975 SIM data problem
Posted: Wed Jun 29, 2011 9:03 pm
by tlahren
Can you give some more information?
- Did it start acting up before you cleaned it or after?
- What is your EM volt reading in your last tune?
- Are you adding any voltage to EM during your method?
- Do you use Alumina to clean the source?
- How does your most recent Tune compare to an older one?
- What is the air and water amounts from the tune?
Re: 5975 SIM data problem
Posted: Wed Jun 29, 2011 9:17 pm
by cjm
EM volts are at 1118 with no added voltage. Air and water checks are well below 10%, I don't have the exact numbers in front of me. I'll have to compare the tune to an older one, but nothing stands out. Also, this mass spec is only 6 months old, and no "samples" have been run. I'm still in method development stage right now. I was collecting full scan and sim data, sometimes it would affect just scan, other times both scan and sim. We are not collecting scan data right now (with the run I showed...). yes to using alumina. yes this was happening before we cleaned..
Re: 5975 SIM data problem
Posted: Wed Jun 29, 2011 9:29 pm
by tlahren
To prove it's the MS try running at least 10 solvent blanks (hexane or isooctane) in a row using an isocratic full scan method where the GC oven only reaches about 100°C for about 60min (or as long as your typical run is).
What exactly do you mean when you say air and water are well below 10%? Is 18 m/z (H2O) above 2% of 69 m/z? On my brand new 5975C (less than 6 months old) in the tune file we have:
H2O @ 1.8%
N2 @ 0.88%
O2 @ 0.7% relative to peak 69 m/z (base).
Are you in the USA? I would call the service Tech who installed it and ask some questions as well. They usually have a pretty good idea as they have seen almost everything.
Re: 5975 SIM data problem
Posted: Wed Jun 29, 2011 9:49 pm
by tlahren
Christian,
I was reading your previous post about LVI/PTV injection reproducibility on PCB homologues. I was wondering why it is necessary for a LVI of PCBs at those detection levels? Currently we run 209 PCBs on a 6890/5973 with the standard SSL injector and a 1 µL injection. Our calibration range for most of the congeners is 0.5 to 1000 ppb. We are using method 1668B (not a HRMS version obviously). I think we are using a 1.5 min SL time on a 300°C SSL injector with a standard gooseneck liner (no wool). I would imagine (using hexane or iso-octane) that you could inject up to 2 µL into this setup and acheive slightly better sensitivity than we have using the 5975C system you have. I also have a 5975C right next to the 73 and the sensitivity differences are incredible. If you are doing LVI injections you should try to run no-injection blanks (system blanks) using your current full scan method. Chose manual injection instead of ALS in the method setup and just hit the "START" button on the front of the machine to start each analysis. It takes some time but it can help answer some questions.
Hope I'm not running you in circles.
Re: 5975 SIM data problem
Posted: Wed Jun 29, 2011 9:56 pm
by cjm
no this is good. thanks for the feedback. i need as much info as possible for these two instruments. I'm running homologs on one instrument and congeners on the other. do you use CI or EI MS? It surprises me you get adequate response for the congeners down to 0.5ppb with a 1uL injection and the range of 0.5 to 1000 ppb stays linear?? our current setup I'm seeing responses of only a few hundred area counts for the 1ppb...and that's 20uL sample injected...I will try the blank runs tomorrow. that's a great idea. thanks.
Re: 5975 SIM data problem
Posted: Wed Jun 29, 2011 10:25 pm
by tlahren
I forgot one probably major detail that is the main factor between our sensitivity differences. We are using a large 6mm drawout plate in an "INERT" ion source instead of the standard 3mm one. In a nutshell it gives better sensitivity and better linearity for the PCBs. There can be other drawbacks with using it but I will let you read the Agilent article about it if you are interested. A buddy of mine switched to this setup on a VOCs instrument and saw a dramatic difference (a good one) in the extended linearity for methylene chloride on his VOC system. If you are interested in this here is the Agilent tech note on it. I know there is another Agilent tech note out there on it but I can't find it right now.
Two main features that can dramatically increase the sensitivity on the 5973/75 systems is the use of an "Inert Source" (all source parts inside have a small "dot" indentation on them) and the 6mm draw-out plate.
http://www.chem.agilent.com/Library/Sup ... a20917.pdf
Re: 5975 SIM data problem
Posted: Fri Jul 01, 2011 2:12 pm
by cjm
I have since pulled the pcb column (rtx-pcb 60m) for a rtx-5sil ms 30m ..trying also to develop a PAH method. I still get incredible noise on simple solvent blanks, when I click anywhere on the chromatogram, EVERY ion is accounted for. There's something very wrong with the mass spec. I think it's time I called agilent. I will run "blank" runs today to take the injection out of the equation.
Re: 5975 SIM data problem
Posted: Thu Aug 18, 2011 10:32 pm
by cjm
Our GC guru has agreed there was a problem with the EM. An autotune run with transfer line capped showed 672 peaks. EM has been replaced. I will try running standards tomorrow. To tlahren, do you believe the 6mm drawout plate will give me better sensitity? I am buying one to try. I cannot tolerate injecting 10uL anymore. Samples leave an unknown deposit on the inside of the liner and my closing standard goes crazy, with response of low chlorinated congeners increasing by 50% and heavily chlorinated congeners decreasing by 50%. I am doing a sulfuric acid cleanup on all samples and I'm wondering if a small amount of acid is being injected, thus interfering and throwing out the calibration. Right now I can run 10 or 12 injections and the liner is trash. If the new 6mm plate helps with sensitivity that would be a blessing. My goal is to run 2 picograms on-column. Anyone laughing??? Can this be done??? I am running a 5975C with inert source.
Re: 5975 SIM data problem
Posted: Fri Aug 19, 2011 12:08 am
by willnatalie
What is the m/z range of your SIM? have you seen any tuning issues?
Re: 5975 SIM data problem
Posted: Fri Aug 19, 2011 2:33 pm
by cjm
50 to 550. I ran a dftpp tune yesterday with excellent results. I am running a test right now with a 2ppb pcb homolog standard doing a 1uL injection. Using dftpp tune parameters with EM at 941 peaks are very noisy. I am going to try adjusting the voltage to see if peak response improves. Hopefully noise response doesn't increase as much...
Re: 5975 Sensitivity questions..
Posted: Mon Aug 22, 2011 12:58 pm
by tlahren
Christian,
I run a 0.5 ppb (about 200 counts in SIM, 0.5 pg @ 1µL injection) standard currently as my lowest cal point. A colleague of mine uses a 1 ppb lower cal point on a 5973 with the 6mm drawout plate. I just replaced the factory 3mm drawout plate on our 5975 with a 6mm one and I am seeing about the same results... Make sure you have the "Inert" source as well. It can be identified by the "dot" pressed on each part of the source. It is very noticeable on the drawout plate. An "Inert" drawout plate should be ordered as well (6mm). The Agilent part number is G2589-20045 and the Description is 73 G2860A DrawoutP. It should be about $370 USD. If you don't have the Inert source your sensitivity might not be the same as I am seeing.
Another note: you stated you were doing the acid cleanup; how many time do you run the extract through this procedure? At one point we used to only do it once then run the sample. Later we were told to keep cleaning the extract with new acid until the lower acid fraction was completely clear. We started doing this and had dramatically better cleanup results (takes a lot of time an glassware though).
Ty
Re: 5975 Sensitivity questions..
Posted: Mon Aug 22, 2011 3:33 pm
by cjm
We are running the inert source. I have ordered the inert plate and hope to get it soon. Very interesting about the acid cleanup...even though we are running air samples and wipes there seems to be an incredible amount of garbage getting in the inlet and on the column. I will definitely take the extra time to do multiple cleanups. If I see positive results from that I will be so relieved. I am already having success injecting 1uL, the problem still exists that my inlet setup is a PTV, and with a 150uL internal volume baffled liner and initial inlet pressure 10psi I either have to use cryo and start inlet below 60 (inject=hexane) and ramp to 350 as I am now, or I can start inlet at 120C (without overloading liner) and ramp to 350 quick as a sort of splitless injection..wow so much to try so little time. I have to come up with a serious game plan to iron out the details but seems like I'm on the right track. Thanks so much for your help I can't wait to share my results.
Re: 5975 Sensitivity questions..
Posted: Thu Aug 25, 2011 3:19 pm
by walkerd2
Hi
We are currently analyzing for a select number of PCB congeners from tissue and plasma samples on our 7890A/5975C in SIM mode. Our detection limits have consistently been around 0.1 ppb with a 2 uL injection of standards prepared in hexane. We've been having a few other issues with our system (mainly increasing standard response following running our extracts, which we are currently trying to eliminate) but regardless you should get a decent response around 0.5 ppb with smaller injection sizes. A few things to try adjusting are:
Increasing the gain factor (currently we found 5 to provide a good balance between signal increase over noise increase
Use a pulsed splitless injection when injecting with the ALS
Also, using a sulfuric acid rinse to cleanup our samples we always complete 2 water rinses after to remove any residual acid remaining.
Doug
Re: 5975 Sensitivity questions..
Posted: Thu Aug 25, 2011 5:19 pm
by cjm
I wonder if it is in fact acid that's making its way into the liner. There's definitely a residue inside after doing multiple injections of samples. If a water rinse would help with my failing closing standards I'll be the happiest analyst ever. Can't wait to try it. Thanks.

