Page 1 of 1
Is there [M+2H2O-H] peak?
Posted: Fri Apr 29, 2011 9:11 am
by thichmoithu
I just received the ESI-MS result from my colleague and he said the peak [M+2H2O-H]-. I'm not sure it is correct, is there this kind of fragment? My compound is a flavone glucoside.
Thanks for help.
Re: Is there [M+2H2O-H] peak?
Posted: Mon May 02, 2011 3:37 pm
by Alp
All sorts of adducts with water are possible and common.
Usually adjusting the voltage parameters for the front end of the instrument (between the ion source and the first set of quads, if using a quadrapole) can break up the adduct leaving just the ion of interest. Sometimes the molecule/ion of interest is too fragile and eliminating the adducts from the mass spectra can be difficult without also breaking up the molecule/ion of interest.
Re: Is there [M+2H2O-H] peak?
Posted: Mon May 02, 2011 5:40 pm
by lmh
but possibly not this one. 2 waters each is 36 (2*18), -1 = 35, but 35 is also chloride. Do you have a big +2 peak (about 1/3rd of the height of the base peak)? If so, it may be a chloride adduct.
Re: Is there [M+2H2O-H] peak?
Posted: Tue May 03, 2011 3:16 pm
by thichmoithu
Although there is an isotop ion peak but I don't think the [M+Cl]+ since the calcd [M+Cl] 431.11089 is to far from the found value 431.15534. My compound is C19H24O9, and this is the ESI-MS spectrum in negative mode:
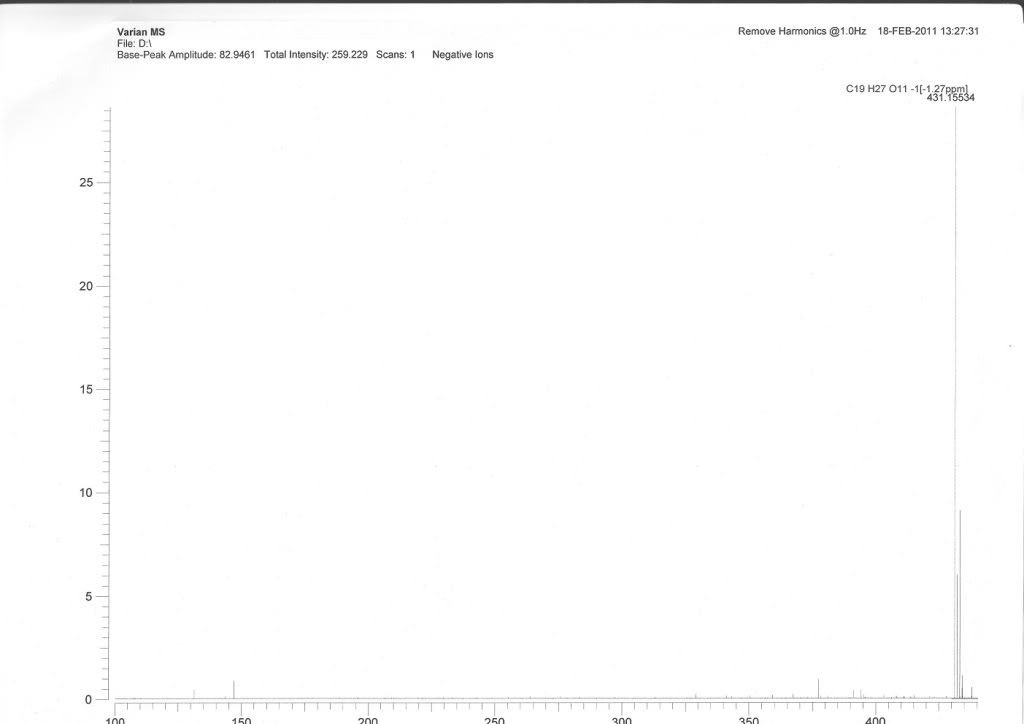
Re: Is there [M+2H2O-H] peak?
Posted: Wed May 04, 2011 7:48 am
by Alp
I did a rough calc
C19H27O11 exact mass 431.1545
C19H24O9 exact mass 396.1413
C19H27O11Cl exact mass 431.1102
Looks like you have an M+2 peak that is about the right size expected for Cl.
What is the mass of that peak?
I am wondering, did you subtract a blank baseline from your scan?
Can the calibration of the instrument be confirmed?
Alp
Re: Is there [M+2H2O-H] peak?
Posted: Wed May 04, 2011 10:05 am
by lmh
yup, you've got a problem: you've got a +2 peak that's the right size for chlorine, but you've got the right mass for addition of two water molecules. Like Alp, I'd also check the calibration before going any further.
Incidentally, in calculating exact masses, it's good practice to allow for the charge, too; an electron does have mass, and it will skew your figures very slightly if you ignore the electron. I think you may have done this? But the mass difference between the expected and measured figures is so big that electrons are a mere detail.
Re: Is there [M+2H2O-H] peak?
Posted: Thu May 05, 2011 9:57 pm
by LC_hoop
Is there any internal standard was added? Without internal standards for calibration, the data with four digital means nothing
