-
- Posts: 83
- Joined: Sat Sep 26, 2009 9:54 am
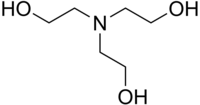
Basically, I've found a certain RP-HPLC method where the following is described : "linear gradient of acetonitrile in triethylammonium phosphate pH 6". Regardeless of the acetonitrile, how is the aqueous phase here prepared? What I have available in my lab is a bottle of triethanol amine, do I use it and adjust pH to 6 with phosphoric acid, or is TEAP purchased already prepared? and how many ml is used? do I have to know the molarity for eg? or is it a default of 1 ml in 1 liter water?
I have googling this topic and found a lot of different methods and left out confused with the above questions, hoping someone bears with me and help me understand this in more detail.