-
- Posts: 8
- Joined: Sun Aug 22, 2010 3:50 pm
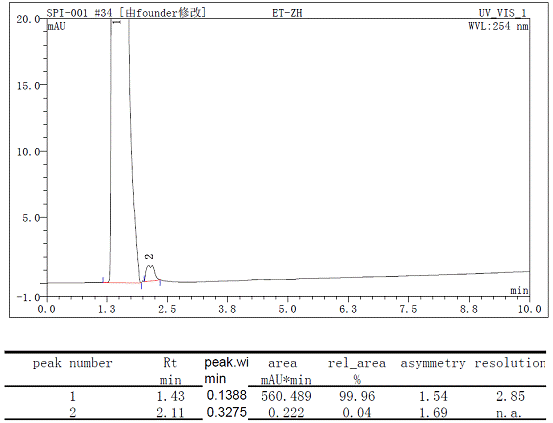
At first, I determined a sample by Shimadzu 10A(made in Japan), I found an odd peak just elute after the main peak, which is about 10%(account by area normalization method)
With same column (C18), mobile phase, temperature and same sample but different Liquid Chromatograph—Dionex U3000, I try it again, the peak just disappear.
I am not sure about the result. So, today I use another chromatograph
(Agilent 1100), and the peak appear again(about 10%)
So I want to know, Does different chromatograph causes different results(I mean the peak numbers)?
Anyone help me?
Gradient
Time ACN(0.1%TFA) Water(0.1%TFA)
0 35% 65%
10 47% 53%
10.1 35% 65%
15 35% 65%
shimadzu chromatogram
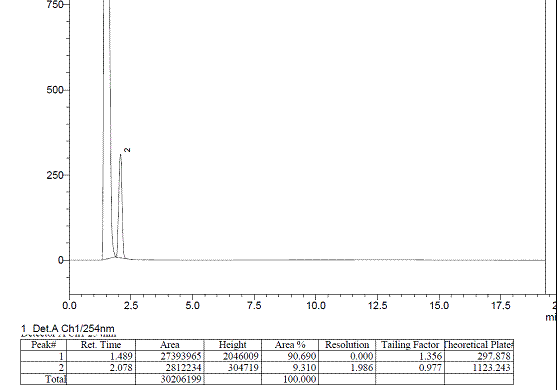
Dionex chromagogram