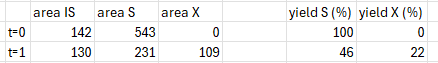How to use relative response factor in
Posted: Wed Nov 20, 2024 10:26 pm
Hi all,
I would like to know how to properly use the Relative Response Factors (RRF) calculated in the following reference (https://analyticalsciencejournals.onlin ... .201500106)
In my case, yield of reactions are calculated using the following formula:

If the product X is the same as the substrate S, no response factor are needed. However if X is another molecule the RRF should be used to correct the yield. This is done as follow:

RRF(IS) does notappear since it would cancel out.
When using it in this manner, I am a bit surprised that when the analyte X is basically double the molar mass of S, the term RFF(s)/RFF(x) is roughly equal to 1.1. I would have expected it to be a larger correction.
Thank you!
I would like to know how to properly use the Relative Response Factors (RRF) calculated in the following reference (https://analyticalsciencejournals.onlin ... .201500106)
In my case, yield of reactions are calculated using the following formula:

If the product X is the same as the substrate S, no response factor are needed. However if X is another molecule the RRF should be used to correct the yield. This is done as follow:

RRF(IS) does notappear since it would cancel out.
When using it in this manner, I am a bit surprised that when the analyte X is basically double the molar mass of S, the term RFF(s)/RFF(x) is roughly equal to 1.1. I would have expected it to be a larger correction.
Thank you!