Page 4 of 6
Re: How to improve purine peak efficency
Posted: Fri Mar 09, 2012 9:40 pm
by Vlad Orlovsky
Good sales people?

Re: How to improve purine peak efficency
Posted: Sat Mar 10, 2012 5:35 am
by carls
IOn the chromatograms that you post Vlad I see that Inosine have a peak shape similar to my shape on kinetex.
I'd open this post to search a way to increase the Inosine peak height at the first time. To increase the less retention problem is the second thing.Thanks!
It seems many have missed the point of the orignal post.
PFP columns were designed to (among other things) increase H-bonding. This may mistakenly be attributed to ion exchange.
Re: How to improve purine peak efficency
Posted: Sat Mar 10, 2012 9:04 am
by danko
What has PFP got to do with free silanols? Or are the latter just a free “side profit” - disigned to broaden the peaks a little bit

Best Regards
Re: How to improve purine peak efficency
Posted: Sat Mar 10, 2012 7:26 pm
by unmgvar
alemaggot
do you really have no other column in the lab?
from my chromsword modelling the inosine is the most polar here and comes of at the void, even while using a polar column or the PFP. it is possible to separate from the 2 other compounds, while they are also quite polar's
HILIC or mixed column will do the work here i think because of the ribose ring in the inosine,
sugars are polar always and come of the void in rp columns,
Re: How to improve purine peak efficency
Posted: Mon Mar 12, 2012 8:56 pm
by Vlad Orlovsky
Here is retention of inosine on Primesep 100 column with sulfuric and formic acids in the mobile phase. Ion-exchange is much weaker than for adenosine (if you can even call it "ion-exchange"):
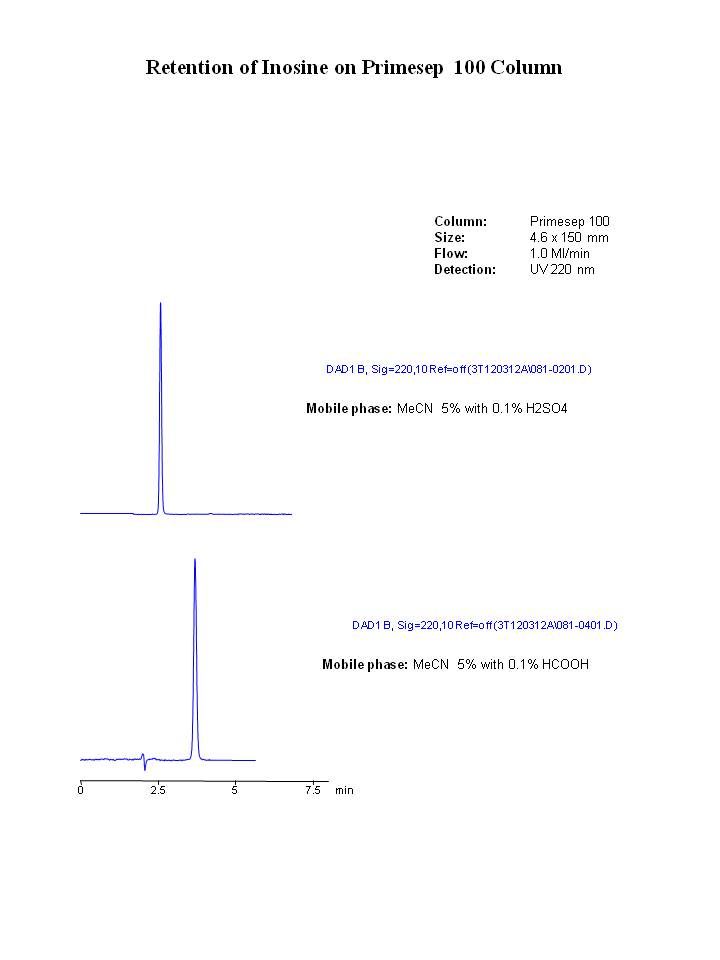
Re: How to improve purine peak efficency
Posted: Tue Mar 13, 2012 8:36 pm
by alemaggot
Hi Vlad,
this chromatogram "say" that the Inosine retention dosen't change whit cation exchange column from C18 column? Right?
For analyze Inosine I think that I'd must work with high pH mobile phase on polyvinil matrix. What you think?
However I've improved my method efficency. I've used a mobile phase with pH 3,0 and phospate buffer instead pH 2.0 with TFA. in this mode I've decrase the retention and the peak height of 4-acetamidobenzoic acid. Now the peak shape of this compound is not perfect. But now I can work with a gradient elution that don't need a high concentration of organic phase and the peak height of my two compound now are plus similar. This is what I've want from the start of my test. Then the similar peak respound! If I can't improve Inosine efficency, I decrease a little the 4-acetamido efficency

Wirh kinetex column I can do a satisificatory separation in less than 10 minutes

When I'll concluse my method development, I'll post some chromatograms!
Re: How to improve purine peak efficency
Posted: Wed Mar 14, 2012 10:58 am
by danko
You maybe realize now that the pH was not as important (within the mentioned range) as the salt in your mobile phase.
Best Regards
Re: How to improve purine peak efficency
Posted: Wed Mar 14, 2012 12:56 pm
by carls
in this mode I've decrase the retention and the peak height of 4-acetamidobenzoic acid. Now the peak shape of this compound is not perfect.
Changing the pH (salt is inconsequential) decreased the retention and symmetry of the 4-acetamidobenzoic acid, i.e. partially ionized. Since nothing was stated about the inosine I assume its behavior remained unchanged (alemaggot, can you comment on the retention and peak shape of inosine with these new conditions?). This does not sound like the best method as slight changes in pH will likely give large changes in the retention of the acid.
Alemaggot - did you try changing the detection wavelength?
Re: How to improve purine peak efficency
Posted: Wed Mar 14, 2012 3:32 pm
by danko
(salt is inconsequential)
Not in relation to free silanols

Best Regards
Re: How to improve purine peak efficency
Posted: Thu Mar 15, 2012 1:19 pm
by carls
Since these analytes are not basic the salt primarily serves to control pH and at this pH any residual silanols have little effect on this separation.
Re: How to improve purine peak efficency
Posted: Thu Mar 15, 2012 11:36 pm
by danko
Since these analytes are not basic
I haven’t checked it but I was under the impression (someone wrote earlier) that at least one of the compounds was an amine. So maybe there's one or maybe more basic compounds in the samples?
at this pH any residual silanols have little effect on this separation.
Since these analytes are not basic the salt primarily serves to control pH and at this pH any residual silanols have little effect on this separation.
Ionized residual silanols at the pH in this context, are absolutely possible. Depending on the residual metals, buried in the silica material, f. ex. the residual silanols could be quite acidic indeed.
Actually there is a test for determining that side of the matter:
Acidic ion exchange capacity at low pH (usually at pH 2.5) – The retention factor ratio between benzylamine and phenol. I'm sure one does perform at Phenomenex a similar test!? So, no exotic thoughts here

Best Regards
Re: How to improve purine peak efficency
Posted: Fri Mar 16, 2012 1:58 am
by carls
key statement: "at this pH any residual silanols have little effect on this separation".
One compound is essentially neutral and the other acidic. Residual silanols will only impact the acidic compound in this separation if there is ion exclusion. At this pH, ionization of residual silanols will be suppressed and thus ion exclusion will not play a significant role in this separation.
Re: How to improve purine peak efficency
Posted: Fri Mar 16, 2012 8:31 am
by danko
Parhaps you missed this:
Ionized residual silanols at the pH in this context, are absolutely possible. Depending on the residual metals, buried in the silica material, f. ex. the residual silanols could be quite acidic indeed.
Actually there is a test for determining that side of the matter:
Acidic ion exchange capacity at low pH (usually at pH 2.5) – The retention factor ratio between benzylamine and phenol. I'm sure one does perform at Phenomenex a similar test!? So, no exotic thoughts here
Best Regards
Re: How to improve purine peak efficency
Posted: Fri Mar 16, 2012 1:02 pm
by alemaggot
There is no change in Inosine peak shape. When I'll can (probably in monday) I'll write the retention time of Inosine at this condition.
I didnt' try to change the detector wavelenght. But I'll can't do some test before the end of march.I'll can do something on this method only after april

best regards
Re: How to improve purine peak efficency
Posted: Fri Mar 16, 2012 1:31 pm
by carls
Danko:
I agree some silanols will be ionized at this pH.
However, the only effect they can have on THIS separation will be through ion exclusion.
Those few silanols that are ionized at pH 4 will not affect THIS separation.
Phenomenex is well aware of the various tests for residual silanols and reports the results of at least one such test on the C of A accompanying the column .


