-
- Posts: 775
- Joined: Mon Dec 01, 2008 9:59 pm
HELIX Chromatography
My opinions might be bias, but I have about 1000 examples to support them. Check our website for new science and applications
www.helixchrom.com
Advertisement
Discussions about HPLC, CE, TLC, SFC, and other "liquid phase" separation techniques.
It seems many have missed the point of the orignal post.IOn the chromatograms that you post Vlad I see that Inosine have a peak shape similar to my shape on kinetex.
I'd open this post to search a way to increase the Inosine peak height at the first time. To increase the less retention problem is the second thing.Thanks!
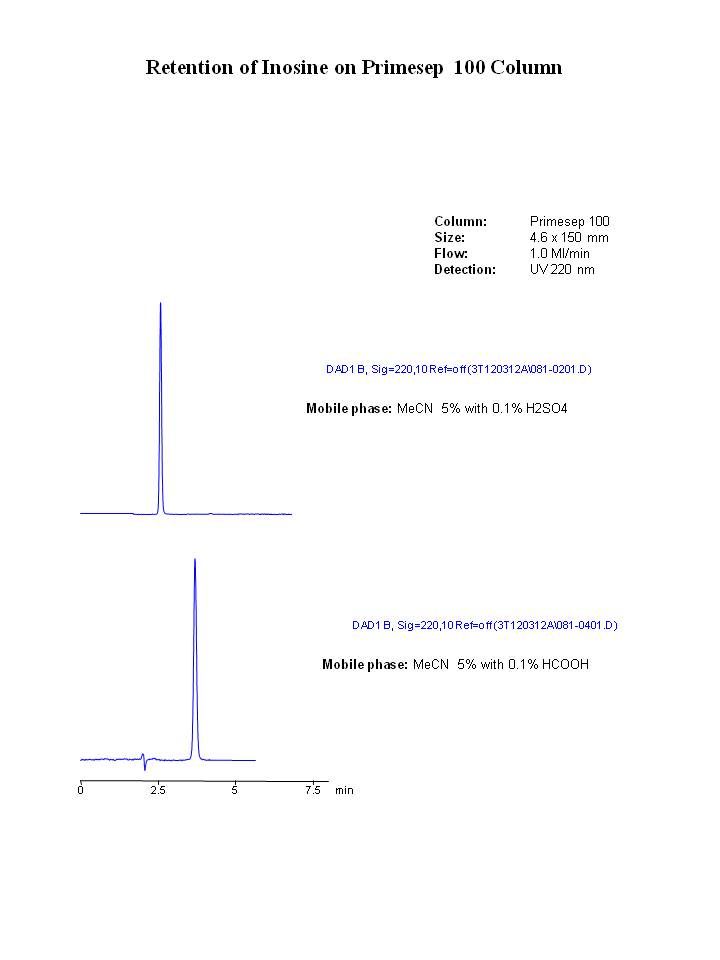
Changing the pH (salt is inconsequential) decreased the retention and symmetry of the 4-acetamidobenzoic acid, i.e. partially ionized. Since nothing was stated about the inosine I assume its behavior remained unchanged (alemaggot, can you comment on the retention and peak shape of inosine with these new conditions?). This does not sound like the best method as slight changes in pH will likely give large changes in the retention of the acid.in this mode I've decrase the retention and the peak height of 4-acetamidobenzoic acid. Now the peak shape of this compound is not perfect.
Not in relation to free silanols(salt is inconsequential)
I haven’t checked it but I was under the impression (someone wrote earlier) that at least one of the compounds was an amine. So maybe there's one or maybe more basic compounds in the samples?Since these analytes are not basic
Ionized residual silanols at the pH in this context, are absolutely possible. Depending on the residual metals, buried in the silica material, f. ex. the residual silanols could be quite acidic indeed.at this pH any residual silanols have little effect on this separation.
Since these analytes are not basic the salt primarily serves to control pH and at this pH any residual silanols have little effect on this separation.
Best RegardsIonized residual silanols at the pH in this context, are absolutely possible. Depending on the residual metals, buried in the silica material, f. ex. the residual silanols could be quite acidic indeed.
Actually there is a test for determining that side of the matter:
Acidic ion exchange capacity at low pH (usually at pH 2.5) – The retention factor ratio between benzylamine and phenol. I'm sure one does perform at Phenomenex a similar test!? So, no exotic thoughts here
Separation Science offers free learning from the experts covering methods, applications, webinars, eSeminars, videos, tutorials for users of liquid chromatography, gas chromatography, mass spectrometry, sample preparation and related analytical techniques.
Subscribe to our eNewsletter with daily, weekly or monthly updates: Food & Beverage, Environmental, (Bio)Pharmaceutical, Bioclinical, Liquid Chromatography, Gas Chromatography and Mass Spectrometry.