Page 3 of 6
Re: How to improve purine peak efficency
Posted: Mon Mar 05, 2012 10:40 pm
by Vlad Orlovsky
found old applications for adenosine in RP/cation-exchange mode. You can see how by changing strength of ionic intercation you can increase retention time:
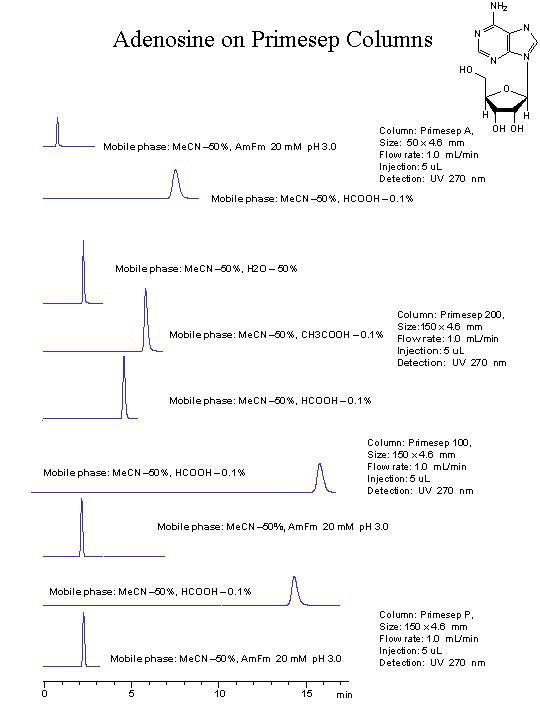
You can also increase retention by reducing amount of ACN from 50% to 5-10%. Retention is controlled by both amount of ACN (RP) and amount of ions/pH (cation-exchange)
Re: How to improve purine peak efficency
Posted: Mon Mar 05, 2012 11:26 pm
by alemaggot
Oooh thank you Vlad! Thank you very much for your post.
Then... to observe this image carry me to think that the best compromise to peak shape and retention of adenosine is give from the use of Primesep 200 and acetic acid. It's right?
Another thing that I observe is that in all test you use 50% of MeCN in mobile phase. It's high as concentration. In my test if I use more than 10% of it Inosine is poor or not reteined really. This difference is by from cation exchange mode? Can I reproduce this condition on my cloumn/system? With this thing I would can to work in isocratic mode!
I reply to you Danko. I'm the best crhomatographer in my lab. I know that I'm unprepared a lot on many discussion. But nobody born masterly, or not?

I must study more too. I know it!
About column... When I buy it I think that was the best choice. But you and other guys had demonstrate the opposite. Ok. I'm sorry for my error, but I can't go behind to repare to it. I must found the best solution with PFP kinetex column. If you want to help in this my mission, you're welcome

Thanks!
Re: How to improve purine peak efficency
Posted: Mon Mar 05, 2012 11:36 pm
by Vlad Orlovsky
Considering everything in your mixture (Isoprinosine or Methisoprinol), I would go with 5-10% ACN and TFA in the mobile phase on Primesep 100 column. This will allow you to retain and separate inosine, acetamidobenzoic acid and dimethylaminoisopropanol. You will need ELSD for dimethylaminoisopropanol.
Inosine will retain by weak reversed-phase and cation-exchanage mechnaisms
acetamidobenzoic acid will retain by reversed-phase mechanism (at low pH created by TFA it is not inized)
dimethylaminoisopropanol will retain by weak reversed-phase and strong cation-exchange mechanisms.
Unfortunately this is not possible on the column you have, but I think it is better to change column now and redevelop the method now with the goal of separating all components of your formulation.
Re: How to improve purine peak efficency
Posted: Tue Mar 06, 2012 3:18 pm
by carls
Inosine is very polar and there is little you can do to increase its retention in reversed phase mode. Ion paring will not be effective since it is difficult to ionize inosine (requires pH ~1 or pH ~10).
The best you can do with RPLC will be to use gradient elution starting at 100% water with 0.1% phosphoric acid (channel A) and 0% methanol with 0.1% phosphoric acid (channel B). Run 2 blank gradients from 0-50% B prior to running your sample (make 5 injections of sample to check reproducibility).
The column must be re-equilibrated with 5 column volumes of 100% A prior to the next gradient run. Run each analyte separately and post your chromatograms here.
Re: How to improve purine peak efficency
Posted: Tue Mar 06, 2012 3:59 pm
by Vlad Orlovsky
Inosine does not require pH 1 or 10 to be retained by ion-exchange mechanism. How would you explain retention on mixed-mode if it is not ionized? As you can see from my chromatograms you have cation-exchange even at pH 3 and 4 on mixed-mode cation-exchange column with plenty of retention (K'>20 in some chromatograms). Less ions, higher pH - more retention. Mixed-mode basically mimics ion-pairing chromatography. Also in mixed-mode you have synergy of two mechanism.
Re: How to improve purine peak efficency
Posted: Tue Mar 06, 2012 10:30 pm
by carls
If what you say about inosine's ionization in pH 3 and 4 solution is true (which I disgaree with) then this user should have been succesful with the early recommendation by Mattias to use an anionic/acidic ion pairing agent (heptane sulfonate) with acidic mobile phase ~pH2 (0.1% TFA).
The ion pairing agent (heptane sulfonate) did not increase retention.
In the meantime hopefully almegot will try gradient elution starting at 100% aqueous with his RPLC column.
The sample should be dissolved in water (no organic)
Re: How to improve purine peak efficency
Posted: Tue Mar 06, 2012 10:57 pm
by Vlad Orlovsky
Please take my explanation with a grain of salt, and don't consider this as another pitch to promote our columns:
I think that when you have two interactions you enhance each of interactions by having a second one, that is why for example if you have hydrophilic molecule with weak basic group you still can control retention by amount of ACN, pH and amount of buffer. You can run hydrophilic basic compound on RP, and have it close to void even at very low organic, when you switch to RP/cation-exchange you will see effect of ACN even going from 30% to 20%. How would you explain drastic increase of retention for adenosine on chromatograms above, when we simply changed pH and amount of ions in the mobile phase? retention increased several folds for the strongest column.
You are saying that you need to be at pH 1 or 10 to have ion exchange and my example says that you don't.
In mixed-mode chromatography 1+1 is never 2, it can be 3, 4 or any other number (I am talking about strength of interactions)
Re: How to improve purine peak efficency
Posted: Wed Mar 07, 2012 3:32 am
by carls
The pKa of the most basic N of adenosine is ~4 while the pKa of the most basic N of inosine is ~1.
Therefore I believe it is very unlikely that the retention on a mixed mode (RP/cation exchange) column will be any different from what was seen with heptane sulfonate.
A strong anion exchange column has a better chance of working than a cation exchanger.
Re: How to improve purine peak efficency
Posted: Wed Mar 07, 2012 4:01 am
by Vlad Orlovsky
If the most basic pKa is 4 for adenosine, retention at pH 4 should be less than at pH 3 and this is not the case.
Re: How to improve purine peak efficency
Posted: Wed Mar 07, 2012 8:44 pm
by alemaggot
I can't enter in your discussion. I'm not prepared on these points.
I don't know what is the best choice for my analysis. HILIC, cation-exchange, ecc. But one thing I know for certain. I must use the kinetex PFP column. Right or wrong that is it. I must create a best method on this column.

Then I write my considerations about it. On the chromatograms that you post Vlad I see that Inosine have a peak shape similar to my shape on kinetex.
I'd open this post to search a way to increase the Inosine peak height at the first time. The reason is that In my sample Inosine and 4-acetamidobenz are mixed in 1:3 ratio. Then the 4-acet. peak go out of my detector full scale, and Inosine no. I'd hope to create a similar respound to adjust Injection/concentration of my sample solution for the best compromise for the peak height.
To increase the less retention problem is the second thing.
To conluse my discourse. I think that there is not possibility to arrange the 2 componente respound with column change. What you think?
Another question. Can I have it with change detector wavelenght?
About retention problem. Ok With cationexchange column Inosine has good retention. But I don't have it

I must work in other direction. If there is not possibility to improve retention on my clomun I'll work with only water with low pH value (for 4-ac.benz. peak shape and retention) and I use a gradient way. What I can do to reduce classic gradient ghost peak? To use a very smal (5%) MeCN from the start of analysis?
Thanks!
Re: How to improve purine peak efficency
Posted: Wed Mar 07, 2012 10:23 pm
by Vlad Orlovsky
Where this is method heading? Are you going to validate it and use in the future? I ordered inosine and will at this in our lab, once I get some results I can probably send you a demo column.
Re: How to improve purine peak efficency
Posted: Thu Mar 08, 2012 9:22 am
by alemaggot
Where this is method heading? Are you going to validate it and use in the future? I ordered inosine and will at this in our lab, once I get some results I can probably send you a demo column.
Yes. I'll use this method in future.
Thank you Vlad. If you want to try it, try it! And for demo column... we'll speak about it in future! Thank you.
meantime... in my other post one user write that Kinetex columns have a very great number of free silaniols. This information is correct? If it's correct they can distrube Inosine eluition, right?
Re: How to improve purine peak efficency
Posted: Thu Mar 08, 2012 10:31 am
by danko
Maybe it’s a good mixed mode column (cation exchange + reversed phase)?

Best Regards
Re: How to improve purine peak efficency
Posted: Fri Mar 09, 2012 1:36 pm
by carls
I must use the kinetex PFP column. Right or wrong that is it. I must create a best method on this column.

I'd open this post to search a way to increase the Inosine peak height at the first time. The reason is that In my sample Inosine and 4-acetamidobenz are mixed in 1:3 ratio. Then the 4-acet. peak go out of my detector full scale, and Inosine no. I'd hope to create a similar respound to adjust Injection/concentration of my sample solution for the best compromise for the peak height.
To increase the less retention problem is the second thing.
To conluse my discourse. I think that there is not possibility to arrange the 2 componente respound with column change. What you think?
Another question. Can I have it with change detector wavelenght?
Thanks!
The UV max for inosine is ~255nm and the UV max for 4-acetamidobenzoic acid is ~270nm.
Using 255-260nm should give the maximum inosine signal.
Re: How to improve purine peak efficency
Posted: Fri Mar 09, 2012 6:48 pm
by unmgvar
alemaggot
can you please explain what was the reason you chose to try a PFP column.
the fact is that it is a poor choice for your case; but more importantly i want to understand the reason why?
learning what got you to choose it, I think is an important lesson here that can help least experience analysts.
thank you in advance

