by
qrys » Tue Jun 15, 2010 5:44 pm
Hi, I have 4 figures here:
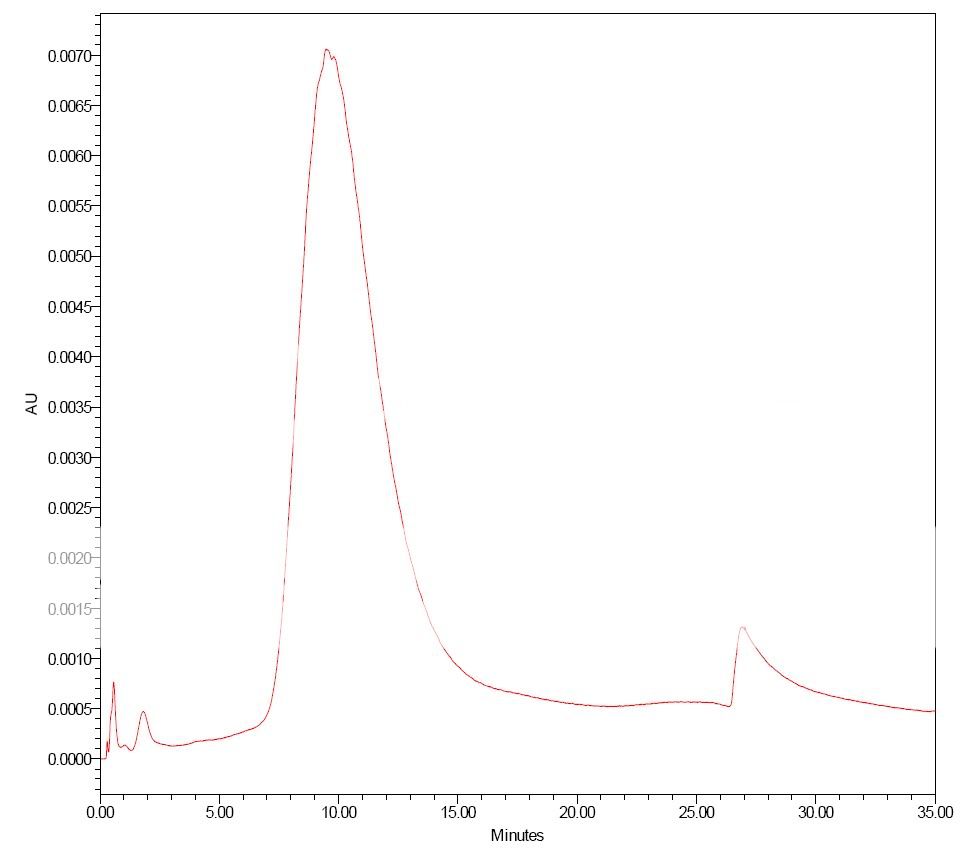
1) isocratic data of lysozyme eluted at various concentration of Na2SO4

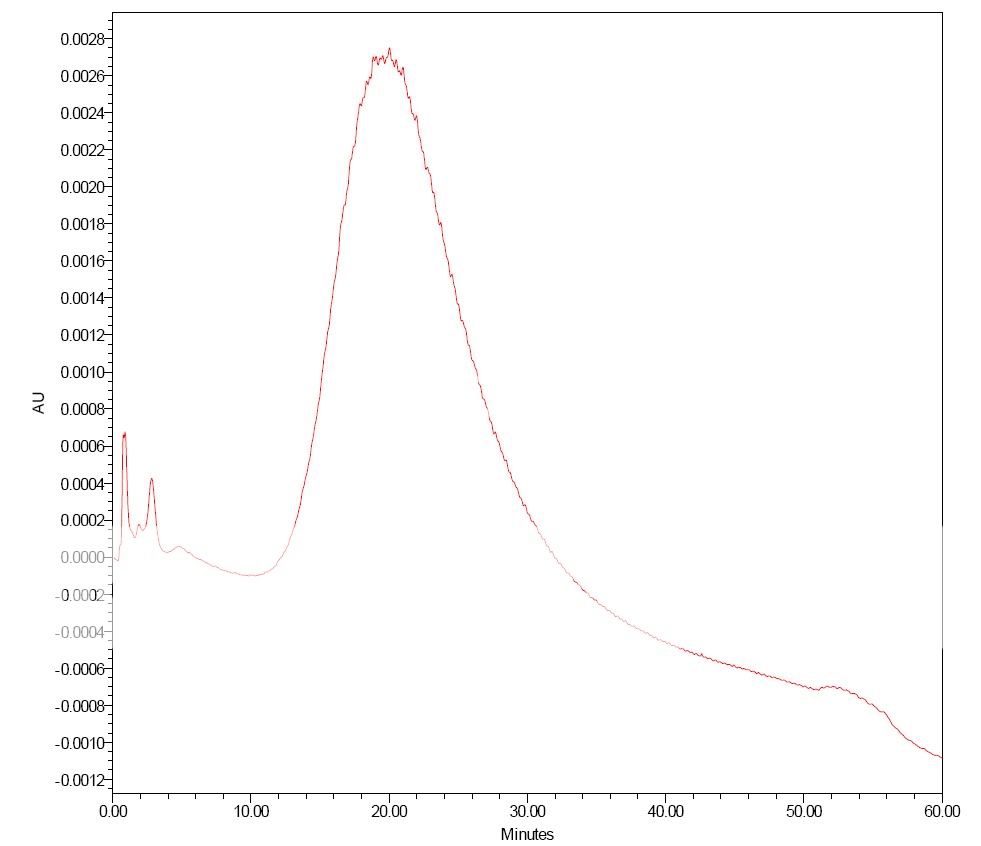
2) chromatogram of lysozyme elution from 50 min gradient of 1 M to 0.5 M Na2SO4, followed by 5 min gradient from 0.5 M to 0 M of Na2SO4. Mobile phase A: 1 M Na2SO4 in phosphate buffer pH 7, B: phosphate buffer pH 7. Sample: lysozyme 5.5 mg/ml in water, 5 ul injection volume.
3) chromatogram of lysozyme elution from 50 min gradient of 1 M to 0.5 M Na2SO4. Mobile phase A: 1 M Na2SO4 in phosphate buffer pH 7 and 10% IPA pH 7, B: phosphate buffer pH 7 with 10% IPA. The chromatogram is not complete 50 min because the lysozyme was eluted very early.
Sample: lysozyme 5.5 mg/ml in water, 5 ul injection volume.
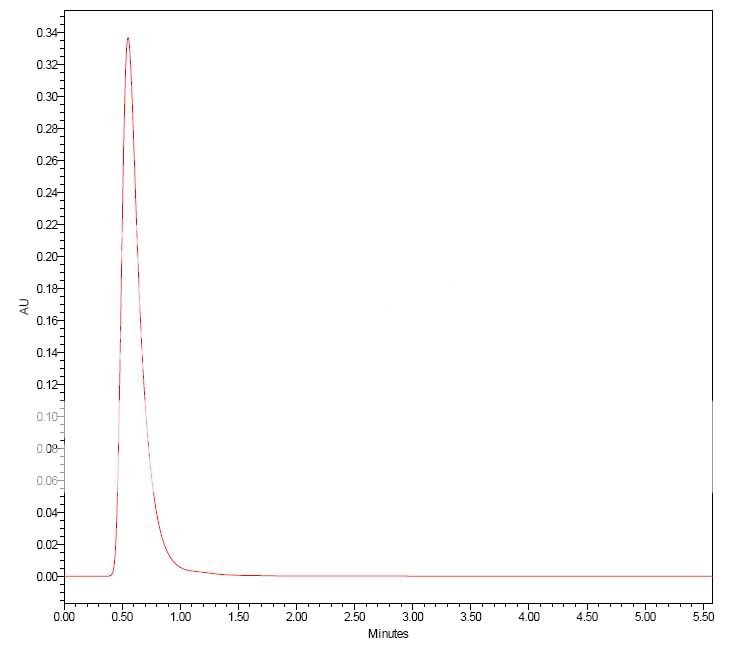
It seems with IPA the sample is almost like unretained?
4) chromatogram of lysozyme elution from 50 min gradient of 1 M to 0.5 M Na2SO4. Mobile phase A: 1 M Na2SO4 in phosphate buffer pH 7 (no IPA). B: phosphate buffer pH 7 with 10% IPA>, this is then followed by isocratic 0.2 M Na2SO4 to remove lysozyme from the column.
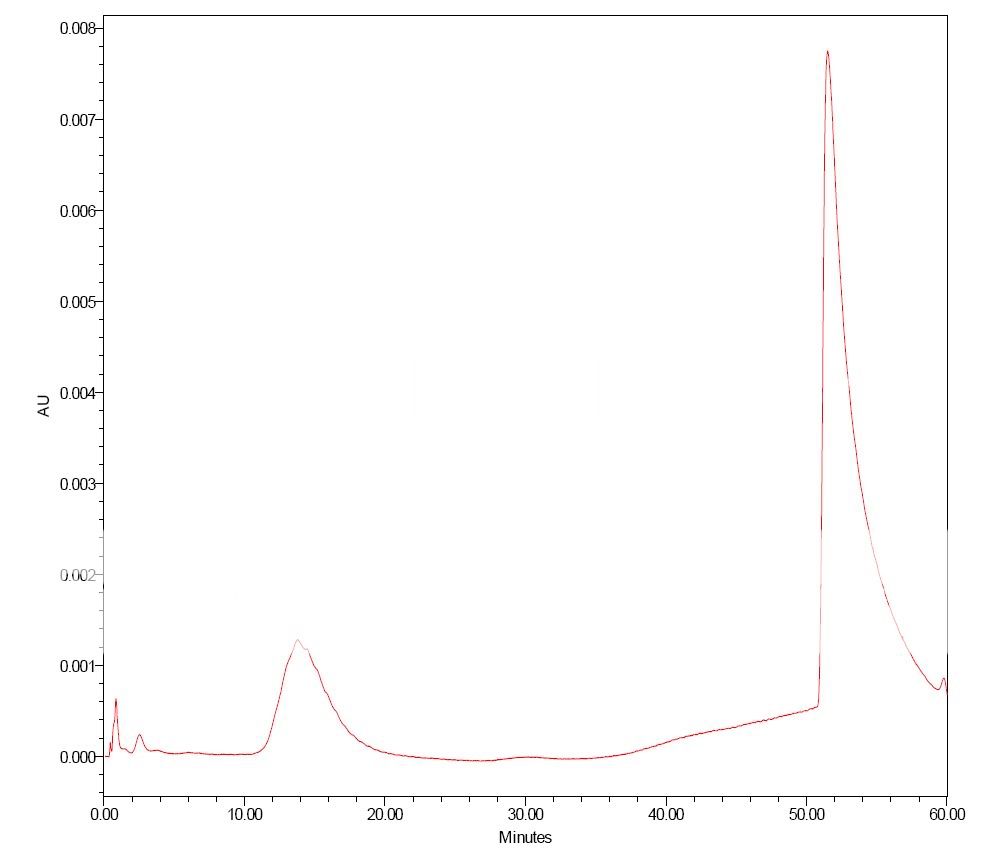
[/img]
Could you explain me the role of IPA here?
Thank you very much, I appreciate you guys are very patient with me