This is an interesting sampling approach for me so I pursued it a little. It might help me on another project.
One of my lab paper towels can take on about 15 mL of liquid - it's pretty saturated by that point but for "proof of concept" it's pretty good. I took 9.5 mL of my 95% EtOH and diluted it to 15 mL with DI water. That's about 60% EtOH in the solution:
9.5 x 0.95 x 100/15 = 60% (ABV)
I added it to a 500 mL beaker and added 1 of my paper towels to the beaker. Allowed the liquid to wick up and saturate the paper towel. I pulled it from the beaker and allowed it to drip until the drips pretty much stopped. It didn't take long. I took 1.0 gram of that saturated paper towel, added it to a 22 mL headspace vial, added 10 mL of acetonitrile and sealed the vial. Shook it for 2-3 minutes. Collected the extract and repeated (2 extracts total).
GC conditions are essentially the same as USP:
https://www.usp.org/sites/default/files ... -m1238.pdf
I've been using a Rtx-1701 (30 m x 0.53 mm x 1.0 µm) because that's what I had. It gives good resolution for their critical analytes.
Analysis of the 2 extracts and using the theory of serial extractions (see any textbook that describes how multiple-headspace-extraction (MHE) works, I see that 90% of the ethanol is removed from the paper in 1 extraction. The sensitivity is such that you could do 2 extractions, pool them, and still detect the ethanol easily.
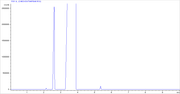
This is the 40 °C isothermal part of the GC analysis. EtOH is easily resolved from the acetonitrile. EtOH is the peak at 2.6 minutes.
Once you know the concentration of EtOH in the acetonitrile extract you know the mass of EtOH in the extract. Once you know the mass in the extract, it is the mass that's in the sample size of wipe you have.
Good luck! This approach will help me sample some aqueous ethanol samples that have too much ethanol in them to get it by headspace SPME (my go-to for volatiles).