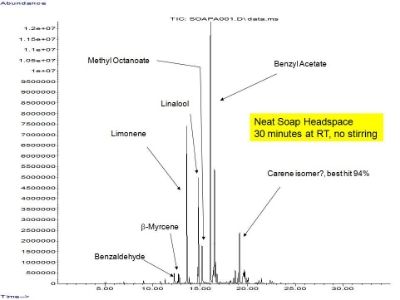
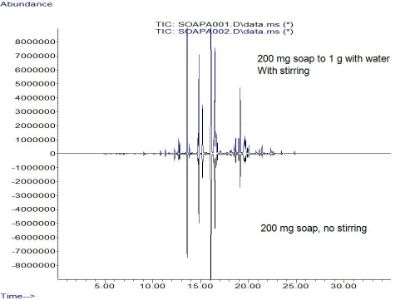
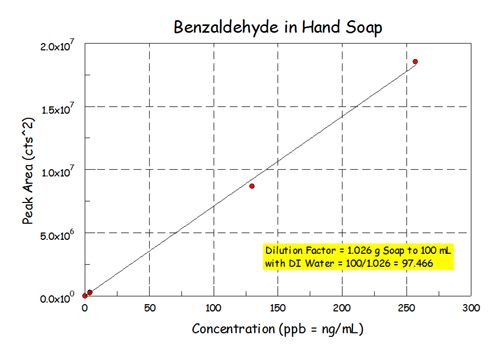
-
- Posts: 1
- Joined: Thu Mar 27, 2014 12:12 pm
I am trying to analyze fragrance mixtures in some of lotions / moisturizers. I have been done extractions with Pentane and Hexane. I have run the extracted sample in the GCMS but then some of base materials including parabens are also extracted along with the aroma chemicals .. which covers some of the peaks and would not allow me to give readings that I want.
Is there any other ways to do better fragrance extractions from the lotions / moisturizers?