-
- Posts: 8
- Joined: Sat Aug 16, 2014 9:22 pm

Basic questions from students; resources for projects and reports.


tom jupille wrote:
Hint: Resolution is defined as the center-to-center separation between two peaks divided by the average of their baseline widths. If you think about it, that should tell you which plate numbers to use.
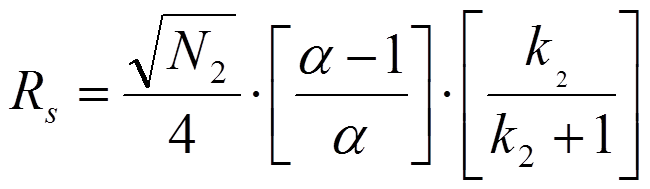
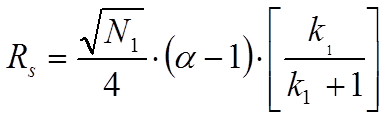
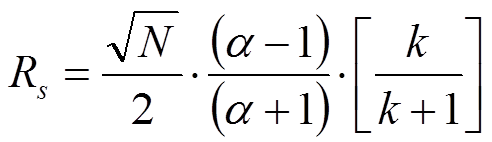
Separation Science offers free learning from the experts covering methods, applications, webinars, eSeminars, videos, tutorials for users of liquid chromatography, gas chromatography, mass spectrometry, sample preparation and related analytical techniques.
Subscribe to our eNewsletter with daily, weekly or monthly updates: Food & Beverage, Environmental, (Bio)Pharmaceutical, Bioclinical, Liquid Chromatography, Gas Chromatography and Mass Spectrometry.