-
- Posts: 73
- Joined: Wed Mar 14, 2012 11:51 am
- Location: Austria
We are using the OPTIC-4 Injector unit from ATAS with our Shimadzu QP2010 ultra.
I am in the middle of method development were i am using splitless injection.
I have some issues with contaminations and I am trying to find the source of the contamination.
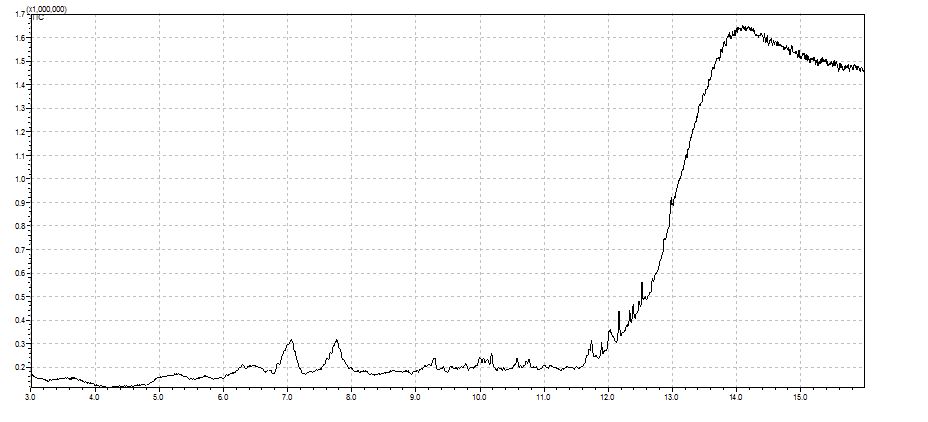
The base line looks fine if I just run the temperature program with no injection.
I get a few contaminant peaks when injection N2 (mostly silanyls)
- tested two different syringes,
- also cleaned syringes with (MeOH GC-Grade, deion H2O HPLC Grade, n-Hexane GC-Grade, Acetone GC-Grade)
When I inject 1 µL MeOH (GC-Grade) i get a lot of contaminant peaks (mostly silanyls but also some others)
- tested different MeOH bottles, all MeOH show the same contaminant peaks
I replaced the liner (A100001) and septa.
In the liner there was clearly some dirt.
Unfortunately with the new liner when running the temperature program (no Injection)
I get now a worse base line than before with the old liner (very noisy, several peaks, and much higher baseline at higher temperatures)
- tried to conditioning the new liner (320°C for 30 min, 0,5 mL/min column flow, 1:100 split ratio, 2.5 mL/min purge flow)
I am desperate and gladly accept any suggestions you have for me.
Mr. Brown