-
- Posts: 2
- Joined: Wed Jan 29, 2020 1:18 pm
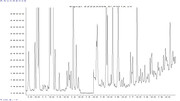
Some background: I am working with BVOC emissions from spruce trees and want to analyse these. To do so I am using adsorbent tubes (collecting volatiles in field) and doing analysis with a TurboMatrix ATD650 coupled with a Shimadzu QP2010 Plus GC-MS system. I ordered and prepared some standards (Isoprene, monoterpenes and sesquiterpenes), which separetes fine and have nice peaks - except for the sesquiterpenes longifolene and beta-caryophyllene which is co-eluting in my chromatogram. So this means that I cannot identify these compounds in my samples, leaving me with three options:
1) Switch column (not optimal due to delivery times and cost)
2) Change the method for my current column (BPX5, 50m, 0.32 mm, 1.0 um)
3) Ignore and analyse the two compounds combined
So my main question is to get advice on what to do - and I also wonder if it actually is possible choose option 2 and change the method? I currently run the following program on the GC-MS: starting temperature at 40 C for 1 min, increase to 210 C with 5 C intervals and once there increasing to 250 C with 20 C interval and hold for 2 minutes. The total time is approx 39 minutes. The column flow is 0.71 mL/min.
Since I dont have that much support at our department (no lab manager etc) I hope some of you might be able to help. Thanks!