I don't have any trouble with these analytes when I use SPME to sample the headspace. My advice is that you should use a wax phase because as a general rule, the carboxylic acids behave badly on non-polar phases. Alcohols chromatograph nicely on this phase as well.
Is the water medium acidic or do you have the ability to add acid to your sample prior to headspace analysis? You don't want to ever add something if it's going to have an adverse effect on your analysis in some other way. If caprylic acid is what controls the pH of the solution, at 10 ppm (6.93 x 10^-5 M) about 34% of it will be ionized to form the conjugate base which will raise your detection limit for this molecule. Adding acid will ensure that you can get the maximum amount of acid into the headspace. If the pH is higher than 5.2 or 5.3, your probability of success is going to go down fairly dramatically.
I was curious about the ethanol part so I did a quick experiment. I added 1.4 µL of 190 proof ethanol (density is 0.789 g/mL) to 100 mL of water and ran it with my "starting point" SPME method. I was able to see EtOH with a flame detector at 10.5 ppm.
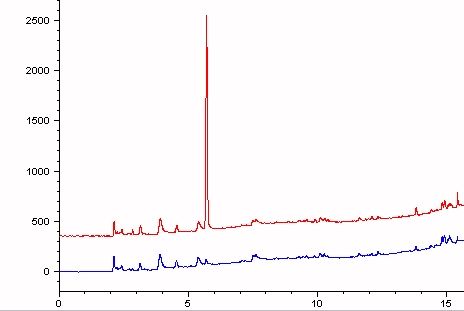
The S/N ratio for that response is 294. If you take the LOQ as the concentration where the S/N ratio is 10, then I can quantitate EtOH at 0.4 ppm. Likewise if the LOD is the concentration that gives a response where the S/N ratio is 3, then the LOD is 0.1 ppm. Not bad for something so water soluble. The split ratio used here is 5:1. I could improve the detection limit if I didn't split the injection as dramatically.
The peak at 5.6 minutes is ethanol. The blue trace is the water blank used to make the standard.
SPME Conditions: Carboxen/PDMS/DVB-2cm, 60 °C preheat for 15 min., followed by 30 minutes headspace sampling at 60 °C. I used a dry-bath heater for this work.
Column: Zebron-Wax (30 m x 0.53 mm x 1.0 µm) from Phenomenex.
Inlet: 230 °C, head pressure 2.5 psig, 5:1 split ratio, helium carrier gas
Oven: 40 °C for 2 min., 40-230 °C at 10 °C/min., 230 °C for 14 min.
Detector: FID, 230 °C, 30:300:25 H2:Air:He makeup
I was able to detect 10.5 ppm EtOH using headspace sampling at room temperature as well - although the detection limit is not as good as when I heated it.
I believe you can do what you desire in this case if you sample with SPME.

